CGS-21680

 | |
| Names | |
|---|---|
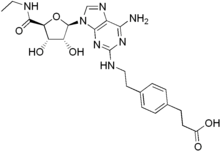
| IUPAC name 3-[4-(2-{[6-Amino-9-(N-ethyl-β-D-ribofuranosyluronamide)-9H-purin-2-yl]amino}ethyl)phenyl]propanoic acid | |
| Systematic IUPAC name 3-{4-[2-({6-Amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxyoxolan-2-yl]-9H-purin-2-yl}amino)ethyl]phenyl}propanoic acid | |
| Other names CGS 21680 | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C23H29N7O6 | |
| Molar mass | 499.52 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
CGS-21680 is a specific adenosine A2A subtype receptor agonist. It is usually presented as an organic hydrochloride salt with a molecular weight of 536.0 g/M. It is soluble up to 3.4 mg/mL in DMSO and 20 mg/mL in 45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin.
The chemical is currently used by researchers interested in studying neuronal transmission with a high-affinity, subtype specific analogue for adenosine. This includes research in respiration where it is believed that A2A receptors are involved in rhythm generation in the pre-Bötzinger complex. The drug is not currently approved for use in a therapeutic capacity.
See also
[edit]References
[edit]- Mayer CA, Haxhiu MA, Martin RJ, Wilson CG (2006). "Adenosine A2A receptors mediate GABAergic inhibition of respiration in immature rats". J Appl Physiol. 100 (1): 91–97. doi:10.1152/japplphysiol.00459.2005. PMID 16141383.
- Xie S, Shafer G, Wilson CG, Martin HB (2006). "In vitro adenosine detection with a diamond-based sensor". Dia Rel Mater. 15 (2–3): 225–228. Bibcode:2006DRM....15..225X. doi:10.1016/j.diamond.2005.08.018.