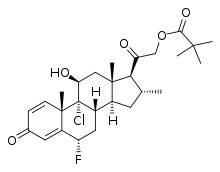
Clocortolone pivalate

 | |
| Clinical data | |
|---|---|
| Trade names | Cilder, Cloderm, Purantix |
| Other names | Clocortolone trimethylacetate; CL-68; SH-863; 9α-Chloro-6α-fluoro-11β,21-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 21-pivalate |
| Drug class | Corticosteroid; Glucocorticoid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.047.099 |
| Chemical and physical data | |
| Formula | C27H36ClFO5 |
| Molar mass | 495.03 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clocortolone pivalate (brand names Cilder, Cloderm, Purantix), also known as clocortolone trimethylacetate, is a synthetic glucocorticoid corticosteroid and corticosteroid ester which is marketed in the United States and Austria.[1][2][3] It is the C21 pivalate (trimethylacetate) ester of clocortolone,[1][2] and acts as a prodrug of clocortolone in the body.[4][5]
References
[edit]- ^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 293–. ISBN 978-1-4757-2085-3.
- ^ a b Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 253–. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 79–. ISBN 978-94-011-4439-1.
- ^ US 5073641, Bundgaard H, Nielsen NM, "Prodrug derivatives of carboxylic acid drugs", issued 17 December 1991
- ^ Stella V, Borchardt R, Hageman M, Oliyai R, Maag H, Tilley J (12 March 2007). Prodrugs: Challenges and Rewards. Springer Science & Business Media. pp. 220–. ISBN 978-0-387-49782-2.