Thulium(III) oxide

 | |
| Names | |
|---|---|
| IUPAC name Thulium(III) oxide | |
| Other names Thulium oxide, thulium sesquioxide | |
| Identifiers | |
3D model (JSmol) | |
| ECHA InfoCard | 100.031.670 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| Tm2O3 | |
| Molar mass | 385.866 g/mol |
| Appearance | greenish-white cubic crystals |
| Density | 8.6 g/cm3 |
| Melting point | 2,341 °C (4,246 °F; 2,614 K) |
| Boiling point | 3,945 °C (7,133 °F; 4,218 K) |
| Solubility | Slightly soluble in acids |
| +51,444·10−6 cm3/mol | |
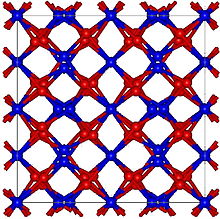
| Structure | |
| Cubic, cI80[1] | |
| Ia-3, No. 206[1] | |
a = 10.49 Å[1] | |
Formula units (Z) | 16[1] |
| Thermochemistry | |
Heat capacity (C) | 2.515 °Cp[2] (25 °C) |
| Hazards | |
| GHS labelling: | |
 | |
| Safety data sheet (SDS) | Sigma-Aldrich |
| Related compounds | |
Other anions | Thulium(III) chloride |
Other cations | Erbium(III) oxide Ytterbium(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Thulium(III) oxide is a pale green crystalline compound, with the formula Tm2O3. It was first isolated in 1879, from an impure sample of erbia, by Swedish chemist Per Teodor Cleve, who named it thulia.
Synthesis
[edit]Thulium(III) oxide has been made in the laboratory using various methods. One method involves burning thulium metal or its various salts in air.[3][2]
Thulium(III) oxide can be made using a hydrothermal method where thulium(III) acetate is mixed with an ammonia solution, which causes thulium(III) oxide to precipitate as a white solid.[1]
Properties
[edit]Thulium(III) oxide (Tm₂O₃) is a pale green, thermally stable powder with a high melting point of 2,341 °C and a density of 8.6 g/cm³, typically forming a cubic crystal structure.[4] It is resistant to oxidation and dissolves in strong acids like hydrochloric acid, allowing it to form soluble thulium salts.[5] Due to its unique f-electron configuration, Tm₂O₃ has notable optical properties.[6] Thulium oxide (Tm₂O₃) is considered fibrogenic; it has the potential to induce tissue injury and fibrosis when inhaled or otherwise introduced to biological tissue.[7]
References
[edit]- ^ a b c d e Lee, Sung Woo; Park, Seong Kyun; Min, Bong-Ki; Kang, Jun-Gill; Sohn, Youngku (July 2014). "Structural/spectroscopic analyses and H2/O2/CO responses of thulium(III) oxide nanosquare sheets". Applied Surface Science. 307: 736–743. doi:10.1016/j.apsusc.2014.04.149.
- ^ a b Justice, Bruce; Westrum, Edgar; Chang, Elfreda; Radebaugh, Ray (February 1, 1969). "Thermophysical properties of the lanthanide oxides. IV. Heat capacities and thermodynamic properties of thulium(III) and lutetium(III) oxides. Electronic energy levels of several lanthanide(III) ions". Journal of Physical Chemistry. 2 (73): 333–340 – via ACSPublications.
- ^ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 25: The f-block metals: lanthanoids and actinoids". Inorganic Chemistry, 3rd Edition. Pearson. p. 864. ISBN 978-0-13-175553-6.
- ^ Loewen, Eric. "Thulium Oxide: Properties and Applications of This Rare Earth Compound". Standford Advanced Materials. Retrieved Oct 30, 2024.
- ^ Mitrovic, I.Z.; Hall, S (2015). "Atomic-layer deposited thulium oxide as a passivation layer on germanium". Journal of Applied Physics. 117: 21404. doi:10.1063/1.4922121.
- ^ Chaneliere, T; Ruggiero, J (2008). "Tm3+:Y2O3 investigated for a quantum light storage application". Physics Review. 77: 245127. doi:10.1103/PhysRevB.77.245127.
- ^ "PubChem Compound Summary for CID 159411, Thulium oxide". National Center for Biotechnology Information. 2024. Retrieved Oct 30, 2024.