Batrachochytrium dendrobatidis
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
| Batrachochytrium dendrobatidis | |
|---|---|
 | |
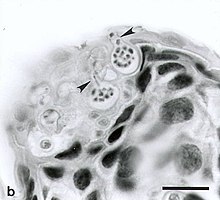
| Zoosporangia of B. dendrobatidis growing on a freshwater arthropod (a) and algae (b); scale bars = 30 µm | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Fungi |
| Division: | Chytridiomycota |
| Class: | Chytridiomycetes |
| Order: | Rhizophydiales |
| Family: | Batrachochytriaceae |
| Genus: | Batrachochytrium |
| Species: | B. dendrobatidis |
| Binomial name | |
| Batrachochytrium dendrobatidis Longcore, Pessier & D.K. Nichols (1999) | |
Batrachochytrium dendrobatidis (/bəˌtreɪkoʊˈkɪtriəm ˈdɛndroʊbətaɪdɪs/ bə-TRAY-koh-KIT-ree-əm DEN-droh-bə-ty-dis), also known as Bd or the amphibian chytrid fungus, is a fungus that causes the disease chytridiomycosis in amphibians.
Since its discovery in 1998 by Lee Berger,[1] the disease devastated amphibian populations around the world, in a global decline towards multiple extinctions, part of the Holocene extinction. A recently described second species, B. salamandrivorans, also causes chytridiomycosis and death in salamanders.
The fungal pathogens that cause the disease chytridiomycosis ravage the skin of frogs, toads, and other amphibians, throwing off their balance of water and salt and eventually causing heart failure, Nature reports. Some amphibian species appear to have an innate capacity to withstand chytridiomycosis infection due to symbiosis with Janthinobacterium lividum. Even within species that generally succumb, some populations survive, possibly demonstrating that these traits or alleles of species are being subjected to evolutionary selection.
Etymology[edit]
The generic name is derived from the Greek words batrachos (frog) and chytra (earthen pot), while the specific epithet is derived from the genus of frogs from which the original confirmation of pathogenicity was made (Dendrobates),[2] dendrobatidis is from the Greek dendron, "tree" and bates, "one who climbs", referring to a genus of poison dart frogs.[3]
Systematics[edit]
Batrachochytrium dendrobatidis was until recently considered the single species of the genus Batrachochytrium. The initial classification of the pathogen as a chytrid was based on zoospore ultrastructure. DNA analysis of the SSU-rDNA has corroborated the view, with the closest match to Chytridium confervae. A second species of Batrachochytrium was discovered in 2013: B. salamandrivorans, which mainly affects salamanders and also causes chytridiomycosis.[4] B. salamandrivorans differs from B. dendrobatidis primarily in the formation of germ tubes in vitro, the formation of colonial thalli with multiple sporangia in vivo, and a lower thermal preference.[4]
Morphology[edit]

B. dendrobatidis infects the keratinized skin of amphibians. The fungus in the epidermis has a thallus bearing a network of rhizoids and smooth-walled, roughly spherical, inoperculate (without an operculum) sporangia. Each sporangium produces a single tube to discharge spores.
Zoospore structure[edit]
Zoospores of B. dendrobatidis, which are typically 3–5 µm in size, have an elongate–ovoidal body with a single, posterior flagellum (19–20 µm long), and possess a core area of ribosomes often with membrane-bound spheres of ribosomes within the main ribosomal mass.[2] A small spur has been observed, located at the posterior of the cell body, adjacent to the flagellum, but this may be an artifact in the formalin-fixed specimens. The core area of ribosomes is surrounded by a single cisterna of endoplasmic reticulum, two to three mitochondria, and an extensive microbody–lipid globule complex. The microbodies closely appose and almost surround four to six lipid globules (three anterior and one to three laterally), some of which appear bound by a cisterna. Some zoospores appear to contain more lipid globules (this may have been a result of a plane-of-sectioning effect, because the globules were often lobed in the zoospores examined). A rumposome has not been observed.[2]
Flagellum structure[edit]
A nonfunctioning centriole lies adjacent to the kinetosome. Nine interconnected props attach the kinetosome to the plasmalemma, and a terminal plate is present in the transitional zone. An inner ring-like structure attached to the tubules of the flagellar doublets within the transitional zone has been observed in transverse section. No roots associated with the kinetosome have been observed. In many zoospores, the nucleus lies partially within the aggregation of ribosomes and was invariably situated laterally. Small vacuoles and a Golgi body with stacked cisternae occurred within the cytoplasm outside the ribosomal area. Mitochondria, which often contain a small number of ribosomes, are densely staining with discoidal cristae.[2]
Life cycle[edit]
B. dendrobatidis has two primary life stages: a sessile, reproductive zoosporangium and a motile, uniflagellated zoospore released from the zoosporangium. The zoospores are known to be active only for a short period of time, and can travel short distances of one to two centimeters.[5] However, the zoospores are capable of chemotaxis, and can move towards a variety of molecules that are present on the amphibian surface, such as sugars, proteins and amino acids.[6] B. dendrobatidis also contains a variety of proteolytic enzymes and esterases that help it digest amphibian cells and use amphibian skin as a nutrient source.[7] Once the zoospore reaches its host, it forms a cyst underneath the surface of the skin, and initiates the reproductive portion of its life cycle. The encysted zoospores develop into zoosporangia, which may produce more zoospores that can reinfect the host, or be released into the surrounding aquatic environment.[8] The amphibians infected with these zoospores are shown to die from cardiac arrest.[9]
Besides amphibians B. dendrobatidis also infects crayfish (Procambarus alleni, P. clarkii, Orconectes virilis, and O. immunis) but not mosquitofish (Gambusia holbrooki).[10]
Physiology[edit]
B. dendrobatidis can grow within a wide temperature range (4-25 °C), with optimal temperatures being between 17 and 25 °C.[11] The wide temperature range for growth, including the ability to survive at 4 °C gives the fungus the ability to overwinter in its hosts, even where temperatures in the aquatic environments are low. The species does not grow well above temperatures of 25 °C, and growth is halted above 28 °C.[11] Infected red-eyed treefrogs (Litoria chloris) recovered from their infections when incubated at a temperature of 37 °C.[12]
Varying forms[edit]
B. dendrobatidis has occasionally been found in forms distinct from its traditional zoospore and sporangia stages. For example, before the 2003 European heat wave that decimated populations of the water frog Rana lessonae through chytridiomycosis, the fungus existed on the amphibians as spherical, unicellular organisms, confined to minute patches (80–120 micrometers across). These organisms, unknown at the time, were subsequently identified as B. dendrobatidis. Characteristics of the organisms were suggestive of encysted zoospores; they may have embodied a resting spore, a saprobe, or a parasitic form of the fungus that is non-pathogenic.[13]
Habitat and relationship to amphibians[edit]
The fungus grows on amphibian skin and produces aquatic zoospores.[14] It is widespread and ranges from lowland forests to cold mountain tops. It is sometimes a non-lethal parasite and possibly a saprophyte. The fungus is associated with host mortality in highlands or during winter, and becomes more pathogenic at lower temperatures.[15]
Geographic distribution[edit]
It has been suggested that B. dendrobatidis originated in Africa or Asia and subsequently spread to other parts of the world by trade in African clawed frogs (Xenopus laevis).[16] In this study, 697 archived specimens of three species of Xenopus, previously collected from 1879 to 1999 in southern Africa, were examined. The earliest case of chytridiomycosis was found in a X. laevis specimen from 1938. The study also suggests that chytridiomycosis had been a stable infection in southern Africa from 23 years prior to finding any infected outside of Africa.[16] There is more recent information that the species originated on the Korean peninsula and was spread by the trade in frogs.[17]
American bullfrogs (Lithobates catesbeianus), also widely distributed, are also thought to be carriers of the disease due to their inherent low susceptibility to B. dendrobatidis infection.[18][19] The bullfrog often escapes captivity and can establish feral populations where it may introduce the disease to new areas.[5] It has also been shown that B. dendrobatidis can survive and grow in moist soil and on bird feathers, suggesting that B. dendrobatidis may also be spread in the environment by birds and transportation of soils.[20] Infections have been linked to mass mortalities of amphibians in North America, South America, Central America, Europe and Australia.[21][22][23] B. dendrobatidis has been implicated in the extinction of the sharp-snouted day frog (Taudactylus acutirostris) in Australia.[24]
A wide variety of amphibian hosts have been identified as being susceptible to infection by B. dendrobatidis, including wood frogs (Lithobates sylvatica),[25] the mountain yellow-legged frog (Lithobates muscosa),[26] the southern two-lined salamander (Eurycea cirrigera),[27] San Marcos Salamander (Eurycea nana), Texas Salamander (Eurycea neotenes), Blanco River Springs Salamander (Eurycea pterophila), Barton Springs Salamander (Eurycea sosorum), Jollyville Plateau Salamander (Eurycea tonkawae),[28] Ambystoma jeffersonianum,[29] the western chorus frog (Pseudacris triseriata), the southern cricket frog (Acris gryllus), the eastern spadefoot toad (Scaphiopus holbrooki), the southern leopard frog (Lithobates sphenocephala),[30] the Rio Grande Leopard frog (Lithobates berlandieri),[31] and the Sardinian newt (Euproctus platycephalus).[32] and endemic frog species, the Beysehir frog in Turkey (Pelophylax caralitanus).[33]
Southeast Asia[edit]
While most studies concerning B. dendrobatidis have been performed in various locations across the world, the presence of the fungus in Southeast Asia remains a relatively recent development. The exact process through which the fungus was introduced to Asia is not known, however, as mentioned above, it has been suggested transportation of asymptomatic carrier species (e.g. Lithobates catesbeianus, the American Bullfrog) may be a key component in the dissemination of the fungus, at least in China.[34] Initial studies demonstrated the presence of the fungus on island states/countries such as Hong Kong,[35] Indonesia,[36] Taiwan,[30] and Japan.[37] Soon thereafter, mainland Asian countries such as Thailand,[38] South Korea,[39] and China[40] reported incidences[spelling?] of B. dendrobatidis among their amphibian populations. Much effort has been put into classifying herpetofauna in countries like Cambodia, Vietnam, and Laos where new species of frogs, toads, and other amphibians and reptiles are being discovered on a frequent basis. Scientists simultaneously are swabbing herpetofauna in order to determine if these newly discovered animals possess traces of the fungus.
In Cambodia, a study showed B. dendrobatidis to be prevalent throughout the country in areas near Phnom Penh (in a village <5 km), Sihanoukville (frogs collected from the local market), Kratie (frogs collected from streets around the town), and Siem Reap (frogs collected from a national preserve: Angkor Centre for Conservation of Biodiversity).[41] Another study in Cambodia questioned the potential anthropological impact in the dissemination of B. dendrobatidis on local amphibian populations in 3 different areas in relation to human interaction: low (an isolated forest atop a mountain people rarely visit), medium (a forest road ~15 km from a village that is used at least once a week), and high (a small village where humans interact with their environment on a daily basis). Using quantitative PCR, evidence of B. dendrobatidis was found in all 3 sites with the highest percentage of amphibians positive for the fungus from the forest road (medium impact; 50%), followed by the mountain forest (low impact; 44%) and village (high impact; 36%).[42] Human influence most likely explains detection of the fungus in the medium and high areas, however it does not provide an adequate explanation why even isolated amphibians were positive for B. dendrobatidis. This may go unanswered until more research is performed on transmission of the fungus across landscapes. However, recent evidence suggests mosquitoes may be a possible vector which may help spread B. dendrobatidis. Another study in French Guiana reports widespread infection, with 8 of 11 sites sampled being positive for B. dendrobatidis infection for at least one species.[43] This study suggests that Bd is more widespread than previously thought.
Effect on amphibians[edit]
Worldwide amphibian populations have been on a steady decline due to an increase in the disease Chytridiomycosis, caused by this Bd fungus. Bd can be introduced to an amphibian primarily through water exposure, colonizing the digits and ventral surfaces of the animal's body most heavily and spreading throughout the body as the animal matures. Potential effects of this pathogen are hyperkeratosis, epidermal hyperplasia, ulcers, and most prominently the change in osmotic regulation often leading to cardiac arrest.[44] The death toll on amphibians is dependent on a variety of factors but most crucially on the intensity of infection. Certain frogs adopt skin sloughing as a defense mechanism for B. dendrobatidis; however, this is not always effective, as mortality fluctuates between species. For example, the Fletcher frog, despite practising skin sloughing, suffers from a particularly high mortality rate when infected with the disease compared to similar species like Lim. peronii and Lim. tasmaniensis. Some amphibian species have been found to adapt to infection after an initial die-off with survival rates of infected and non-infected individuals being equal.[45]
According to a study by the Australian National University estimates that the Bd fungus has caused the decline of 501 amphibian species—about 6.5 percent of the world known total. Of these, 90 have been entirely wiped out and another 124 species have declined by more than 90 percent, and their odds of the effected species recovering to a healthy population are doubtful.[46] However, these conclusions were criticized by later studies, which proposed that Bd was not as primary a driver of amphibian declines as found by the previous study.[47]
One amphibian in particular that Batrachochytrium dendrobatidis (Bd) has affected greatly was the Lithobates clamitans. Bd kills this frog by interfering with external water exchange thereby causing an imbalance with ion exchange which leads to heart failure.
Immunity[edit]
Some amphibian species are actually immune to Bd, or have biological protections against the fungus.[48] One such salamander is the alpine salamander, or S. atra. These salamanders have several subspecies, but they share a common trait: toxicity. A 2012 study demonstrated that no alpine salamanders in the area had the disease, despite its prevalence in the area.[49] Alpine salamanders can produce alkaloid products[50][49] or other toxic peptides[50] that may be protective against microbes.[51]
See also[edit]
References[edit]
- ^ Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H (July 1998). "Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America". Proceedings of the National Academy of Sciences of the United States of America. 95 (15): 9031–6. Bibcode:1998PNAS...95.9031B. doi:10.1073/pnas.95.15.9031. PMC 21197. PMID 9671799.
- ^ a b c d Longcore JE, Pessier AP, Nichols DK (1999). "Batrachochytrium Dendrobatidis gen. et sp. nov, a chytrid pathogenic to amphibians". Mycologia. 91 (2): 219–227. doi:10.2307/3761366. JSTOR 3761366.
- ^ "Etymologia: Batrachochytrium salamandrivorans". Emerg Infect Dis. 22 (7): 1282. July 2016. doi:10.3201/eid2207.ET2207. PMC 4918143.
- ^ a b Martel, A.; Spitzen-van der Sluijs, A.; Blooi, M.; Bert, W.; Ducatelle, R.; Fisher, M. C.; Woeltjes, A.; Bosman, W.; Chiers, K.; Bossuyt, F.; Pasmans, F. (2013). "Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians". Proceedings of the National Academy of Sciences of the United States of America. 110 (38): 15325–15329. Bibcode:2013PNAS..11015325M. doi:10.1073/pnas.1307356110. PMC 3780879. PMID 24003137.
- ^ a b Garner TW, Perkins MW, Govindarajulu P, Seglie D, Walker S, Cunningham AA, Fisher MC (September 2006). "The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Lithobates catesbeiana". Biol. Lett. 2 (3): 455–9. doi:10.1098/rsbl.2006.0494. PMC 1686185. PMID 17148429.[permanent dead link]
- ^ Moss AS, Reddy NS, Dortaj IM, San Francisco MJ (2008). "Chemotaxis of the amphibian pathogen Batrachochytrium dendrobatidis and its response to a variety of attractants". Mycologia. 100 (1): 1–5. doi:10.3852/mycologia.100.1.1. PMID 18488347.
- ^ Symonds EP, Trott DJ, Bird PS, Mills P (2008). "Growth characteristics and enzyme activity in Batrachochytrium dendrobatidis isolates". Mycopathologia. 166 (3): 143–147. doi:10.1007/s11046-008-9135-y. PMID 18568420. S2CID 8545084.
- ^ Berger L, Hyatt AD, Speare R, Longcore JE (December 2005). "Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis". Dis. Aquat. Org. 68 (1): 51–63. doi:10.3354/dao068051. PMID 16465834.
- ^ Voyles J, Young S, Berger L, Campbell C, Voyles WF, Dinudom A, Cook D, Webb R, Alford RA, Skerratt LF, Speare R (2009). "Pathogenesis of chytridiomycosis, a cause of catastrophic amphibian declines". Science. 326 (5952): 582–585. Bibcode:2009Sci...326..582V. doi:10.1126/science.1176765. PMID 19900897. S2CID 52850132.
- ^ McMahon, T. A.; Brannelly, L. A.; Chatfield, M. W.; Johnson, P. T.; Joseph, M. B.; McKenzie, V. J.; Richards-Zawacki, C. L.; Venesky, M. D.; Rohr, J. R. (2013). "McMahon, Taegan A. et al "Chytrid fungus Batrachochytrium dendrobatidis has nonamphibian hosts and releases chemicals that cause pathology in the absence of infection." Proceedings of the National Academy of Sciences 110.1 (2013): 210-215. Web. 01 Nov. 2020". Proceedings of the National Academy of Sciences of the United States of America. 110 (1): 210–5. doi:10.1073/pnas.1200592110. PMC 3538220. PMID 23248288.
- ^ a b Piotrowski JS, Annis S, Longcore JE (2004). "Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians". Mycologia. 96 (1): 9–15. doi:10.2307/3761981. JSTOR 3761981. PMID 21148822.
- ^ Woodhams DC, Alford RA, Marantelli G (June 2003). "Emerging disease of amphibians cured by elevated body temperature". Dis. Aquat. Org. 55 (1): 65–7. doi:10.3354/dao055065. PMID 12887256.
- ^ Di Rosa I, et al. (2007). "The Proximate Cause of Frog Declines?". Nature. 447 (7144): E4–E5. Bibcode:2007Natur.447....4R. doi:10.1038/nature05941. PMID 17538572. S2CID 4421285.
- ^ Ron SR (2005). "Predicting the Distribution of the Amphibian Pathogen B. dendrobatidis in the New World". Biotropica. 37 (2): 209–221. doi:10.1111/j.1744-7429.2005.00028.x. S2CID 84272576.
- ^ Daszak P, Cuningham AA, Hyatt AD (2003). "Infection disease and amphibian population declines". Divers. Distrib. 9 (2): 141–150. doi:10.1046/j.1472-4642.2003.00016.x. S2CID 16838374.
- ^ a b Weldon C, du Preez LH, Hyatt AD, Muller R, Spears R (December 2004). "Origin of the amphibian chytrid fungus". Emerging Infect. Dis. 10 (12): 2100–5. doi:10.3201/eid1012.030804. PMC 3323396. PMID 15663845.
- ^ "Frog-Killing Fungus Found to Have Origins on Korean Peninsula". The New York Times. 2018-05-10. ISSN 0362-4331. Retrieved 2018-05-20.
- ^ Kats LB, Ferrer RP (2003). "Alien predators and amphibian declines: review of two decades of science and the transition to conservation". Diversity and Distributions. 9 (2): 99–110. doi:10.1046/j.1472-4642.2003.00013.x. S2CID 85969277.
- ^ Daszak P, Strieby A, Cunningham AA, Longcore JE, Brown CC, Porter D (2004). "Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians". Herpetological Journal. 14: 201–207.
- ^ Johnson ML, Speare R (July 2005). "Possible modes of dissemination of the amphibian chytrid Batrachochytrium dendrobatidis in the environment" (PDF). Dis. Aquat. Org. 65 (3): 181–6. doi:10.3354/dao065181. PMID 16119886.
- ^ Lips KR (1999). "Mass mortality and population declines of anurans at an upland site in western Panama". Conservation Biology. 13 (1): 117–125. doi:10.1046/j.1523-1739.1999.97185.x. S2CID 86205459.
- ^ Daszak P, Cunningham AA, Hyatt AD (2003). "Infectious disease and amphibian population declines" (PDF). Diversity and Distributions. 9 (2): 141–50. doi:10.1046/j.1472-4642.2003.00016.x. S2CID 16838374. Archived from the original (PDF) on 2008-12-26.
- ^ Herrera RA, Steciow MM, Natale GS (2005). "Chytrid fungus parasitizing the wild amphibian Leptodactylus ocellatus (Anura: Leptodactylidae) in Argentina". Diseases of Aquatic Organisms. 64 (3): 247–52. doi:10.3354/dao064247. PMID 15997823.
- ^ Schloegel LM, Hero JM, Berger L, Speare R, McDonald K, Daszak P (2006). "The decline of the sharp-snouted day frog (Taudactylus acutiostris): the first documented case of extinction by infection in a free-ranging wildlife species?". EcoHealth. 3: 35–40. CiteSeerX 10.1.1.602.3591. doi:10.1007/s10393-005-0012-6. S2CID 11114174.
- ^ Reeves MK (2008). "Batrachochytrium dendrobatidis in wood frogs (Lithobates sylvatica) from Three National Wildlife Refuges in Alaska, USA". Herpetological Review. 39 (1): 68–70.
- ^ Andre SE, Parker J, Briggs CJ (2008). "Effect of temperature on host response to Batrachochytrium dendrobatidis infection in the mountain yellow-legged frog (Lithobates muscosa)". Journal of Wildlife Diseases. 44 (3): 716–720. doi:10.7589/0090-3558-44.3.716. PMID 18689660.
- ^ Byrne MW, Davie EP, Gibbons JW (2008). "Batrachochytrium dendrobatidis occurrence in Eurycea cirrigera". Southeastern Naturalist. 7 (3): 551–555. doi:10.1656/1528-7092-7.3.551. S2CID 84713825.
- ^ Gaertner JP, Forstner MR, O'Donnell L, Hahn D (2009). "Detection of Batrachochytrium dendrobatidis in endemic salamander species from Central Texas". EcoHealth. 6 (1): 20–26. doi:10.1007/s10393-009-0229-x. PMID 19424755. S2CID 23997421.
- ^ Brodman R, Briggler JT (2008). "Batrachochytrium dendrobatidis in Ambystoma jeffersonianum larvae in southern Indiana". Herpetological Review. 39 (3): 320–321.
- ^ a b Lehtinen RM, Kam Y-C Richards CL (2008). "Preliminary surveys for Batrachochytrium dendrobatidis in Taiwan". Herpetological Review. 39 (3): 317–318.
- ^ Lovich R, Ryan MJ, Pessier AP, CLaypool B (2008). "Infection with the fungus Batrachochytrium dendrobatidis in a non-native Lithobates berlandieri below sea level in the Coachella Valley, California, USA". Herpetological Review. 39 (3): 315–317.
- ^ Bovero S, Sotgiu G, Angelini C, Doglio S, Gazzaniga E, Cunningham AA, Garner TW (2008). "Detection of chytridiomycosis caused by Batrachochytrium dendrobatidis in the endangered sardinian newt (Euproctus platycephalus), in Southern Sardinia, Italy". Journal of Wildlife Diseases. 44 (3): 712–715. doi:10.7589/0090-3558-44.3.712. PMID 18689659.
- ^ Erismis UC, Konuk M, Yoldas T, Agyar P, Yumuk D, Korcan SE (2014). "Survey of Turkey's endemic amphibians for chytrid fungus Batrachochytrium dendrobatidis, in Turkey" (PDF). Journal of Wildlife Diseases. 111 (2): 153–157. doi:10.3354/dao02742. PMID 25266902.
- ^ Bai, C.; T. W. Garner & Y. Li (2010). "First evidence of Batrachochytrium dendrobatidis in China: discovery of chytridiomycosis in introduced American bullfrogs and native amphibians in the Yunnan Province, China". Dis Aquat Org. 92 (1): 241–244. doi:10.1007/s10393-010-0307-0. PMID 20372969. S2CID 24321977.
- ^ Rowley J, Chan SK, Tang WS, Speare R, Skerratt LF, Alford RA, Cheung KS, Ho CY, Campbell R (2007). "Survey for the amphibianchytrid Batrachochytrium dendrobatidis in Hong Kong in native amphibians and in the international amphibian trade". Diseases of Aquatic Organisms. 78 (2): 87–95. doi:10.3354/dao01861. PMID 18286805.
- ^ Kusrini MD, Skerratt LF, Garland S, Berger L, Endarwin W (2008). "Chytridiomycosis in frogs of Mount Gede Pangrango, Indonesia" (PDF). Diseases of Aquatic Organisms. 82 (3): 187–194. doi:10.3354/dao01981. PMID 19244970.
- ^ Fisher MC, Garner TW, Walker SF (2009). "Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis inspace, time, and host". Annual Review of Microbiology. 63: 291–310. doi:10.1146/annurev.micro.091208.073435. PMID 19575560.
- ^ McLeod DS, Sheridan JA, Jiraungkoorskul W, Khonsue W (2008). "A survey for chytrid fungus in Thai amphibians". Raffles Bulletin of Zoology. 56: 199–204.
- ^ Yang H; H. Baek; R. Speare; R. Webb; S. Park; T. Kim; K.C. Lasat er; S. Shin; S. Son; J. Park; M. Min; Y. Kim; K. Na; H. Lee & S. Park (2008). "First detection of the amphibian chytrid fungus Batrachochytrium dendrobatidis in free-ranging populations of amphibians on mainland Asia: survey in South Korea". Dis Aquat Org. 86 (1): 9–13. doi:10.3354/dao02098. PMID 19899344.
- ^ Wei, Y.; K. Xu; D.-Z. Zhu; X.-F. Chen & X.-L. Wang (2010). "First Early-spring survey for Batrachochytrium dendrobatidis in wild Rana dybowskii in Heilongjiang Province, China". Dis Aquat Org. 92 (3): 241–244. doi:10.3354/dao02172. PMID 21268987.
- ^ Gaertner JP, Mendoza JA, Forstner MR, Neang T, Hahn D (2011). "Detection of Batrachochytrium dendrobatidis in frogs from different locations in Cambodia". Herpetological Review. 42: 546–548.
- ^ Mendoza JA, Gaertner JP, Holden J, Forstner MR, Hahn D (2011). "Detection of Batrachochytrium dendrobatidis on amphibians in Pursat Province, Cambodia". Herpetological Review. 42: 542–545.
- ^ Courtois EA, Gaucher P, Chave J, Schmeller DS (2015). "Widespread Occurrence of Bd in French Guiana, South America". PLOS ONE. 10 (4): e0125128. Bibcode:2015PLoSO..1025128C. doi:10.1371/journal.pone.0125128. PMC 4406614. PMID 25902035.
- ^ "Chytridiomycosis". www.amphibiaweb.org. Retrieved 2016-05-27.
- ^ DiRenzo, Graziella; Zipkin, Elise; Grant, Evan Campbell; Royle, J. Andrew; Longo, Ana; Zamudio, Kelly; Lips, Karen (3 October 2018). "Eco‐evolutionary rescue promotes host–pathogen coexistence". Ecological Applications. 28 (8): 1948–1962. doi:10.1002/eap.1792. PMID 30368999.
- ^ Yong, Ed (2019-03-28). "The Worst Disease Ever Recorded". The Atlantic. Retrieved 2019-03-28.
- ^ Lambert, Max R.; Womack, Molly C.; Byrne, Allison Q.; Hernández-Gómez, Obed; Noss, Clay F.; Rothstein, Andrew P.; Blackburn, David C.; Collins, James P.; Crump, Martha L.; Koo, Michelle S.; Nanjappa, Priya (2020-03-20). "Comment on "Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity"". Science. 367 (6484): eaay1838. doi:10.1126/science.aay1838. ISSN 0036-8075. PMID 32193293.
- ^ Pereira, K. E.; Woodley, S. K. (2021-01-11). "Skin defenses of North American salamanders against a deadly salamander fungus". Animal Conservation. 24 (4): 552–567. doi:10.1111/acv.12666. ISSN 1367-9430. S2CID 232030040.
- ^ a b R, Lötters, S Kielgast, J Sztatecsny, M Wagner, N Schulte, U Werner, P Rödder, D Dambach, J Reissner, T Hochkirch, A Schmidt, B (2012-04-30). Absence of infection with the amphibian chytrid fungus in the terrestrial Alpine salamander, Salamandra atra. Deutsche Gesellschaft für Herpetologie und Terrarienkunde (DGHT). OCLC 1030045649.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b DE MEESTER, Gilles; ŠUNJE, Emina; PRINSEN, Els; VERBRUGGEN, Erik; VAN DAMME, Raoul (2020-10-13). "Toxin variation among salamander populations: discussing potential causes and future directions". Integrative Zoology. 16 (3): 336–353. doi:10.1111/1749-4877.12492. hdl:10067/1718680151162165141. ISSN 1749-4877. PMID 32965720. S2CID 221862886.
- ^ Lüddecke, Tim; Schulz, Stefan; Steinfartz, Sebastian; Vences, Miguel (2018-09-04). "A salamander's toxic arsenal: review of skin poison diversity and function in true salamanders, genus Salamandra". The Science of Nature. 105 (9–10): 56. Bibcode:2018SciNa.105...56L. doi:10.1007/s00114-018-1579-4. ISSN 0028-1042. PMID 30291447. S2CID 253637272.
Further reading[edit]
- Daszak, Peter; Berger L; Cunningham AA; Hyatt AD; Green DE; Speare R. (1999). "Emerging Infectious Diseases and Amphibian Population Declines". Emerging Infectious Diseases. 5 (6): 735–748. doi:10.3201/eid0506.990601. PMC 2640803. PMID 10603206.
- Johnson, Megan L.; Speare, Richard (August 2003). "Survival of Batrachochytrium dendrobatidis in Water: Quarantine and Disease Control Implications". Emerging Infectious Diseases. 9 (8): 915–921. doi:10.3201/eid0908.030145. PMC 3020615. PMID 12967488.