Dactolisib
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
 | |
| Names | |
|---|---|
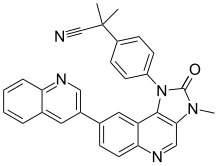
| Preferred IUPAC name 2-Methyl-2-{4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl]phenyl}propanenitrile | |
| Other names NVP-BEZ235; BEZ-235; RTB101 | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C30H23N5O | |
| Molar mass | 469.548 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dactolisib (codenamed NVP-BEZ235 and BEZ-235, also known as RTB101) is an imidazoquinoline derivative acting as a PI3K inhibitor.[1] It also inhibits mTOR.[2] It is being investigated as a possible cancer treatment.[3]
It has been shown to be toxic to Waldenström's macroglobulinemia cells.[4]
It was the first PI3K inhibitor to enter clinical trials, in 2006.[5]
A phase IB/II clinical trial for locally advanced or metastatic HER2 negative breast cancer has completed.[6]
A phase II clinical trial for advanced pancreatic neuroendocrine tumors (pNET) had initially reported results, but was later terminated because insufficient normal tissue tolerance to the drug.[7] A phase I clinical trial of BEZ235 in patients with advanced renal cell carcinoma had to be terminated prematurely due to toxicity and a lack of clinical efficacy .[8] Another Phase Ib study on patients with various solid cancers found severe normal tissue toxicity as well when BEZ235/Dactolisib was administered in combination with the mTOR inhibitor Everolimus. The authors concluded that the combination of both drugs demonstrated limited efficacy and tolerance. BEZ235 systemic exposure increased in a dose-proportional manner while oral bioavailability was quite low, which may be related to gastrointestinal-specific toxicity .[9] A phase I study of BEZ-235 to treat acute lymphoid leukaemia was initiated in 2012, but no results were published since then.[10]
A phase 2a randomized, placebo-controlled clinical trial published in 2018 showed that everolimus in combination with dactolisib decreased the rate of reported infections in an elderly population.[11]
References[edit]
- ^ Liu, TJ; Koul, D; LaFortune, T; Tiao, N; Shen, RJ; Maira, SM; Garcia-Echevrria, C; Yung, WKA (11 August 2009). "NVP-BEZ235, a Novel Dual Phosphatidylinositol 3-kinase/Mammalian Target of Rapamycin Inhibitor, Elicits Multifaceted Antitumor Activities in Human Gliomas". Molecular Cancer Therapeutics. 8 (8): 2204–10. doi:10.1158/1535-7163.MCT-09-0160. PMC 2752877. PMID 19671762.
- ^ Awasthi, N; Yen, PL; Schwarz, MA; Schwarz, RE (March 2012). "The Efficacy of a Novel, Dual PI3K/mTOR Inhibitor NVP-BEZ235 to Enhance Chemotherapy and Antiangiogenic Response in Pancreatic Cancer". Journal of Cellular Biochemistry. 113 (3): 784–91. doi:10.1002/jcb.23405. PMID 22020918. S2CID 23005922.
- ^ Maira, SM; Stauffer, F; Schnell, C; García-Echeverría, C (1 February 2009). "PI3K Inhibitors for Cancer Treatment: Where Do We Stand?". Biochemical Society Transactions. 37 (1): 265–72. doi:10.1042/BST0370265. PMID 19143644.
- ^ Sacco, A; Roccaro, A; Ghobrial, IM (November 2010). "Role of Dual PI3/Akt and mTOR Inhibition in Waldenström's Macroglobulinemia". Oncotarget. 1 (7): 578–82. doi:10.18632/oncotarget.192. PMC 3248138. PMID 21317453.
- ^ "A Phase I/II Study of BEZ235 in Patients with Advanced Solid Malignancies Enriched by Patients with Advanced Breast Cancer". ClinicalTrials.gov. Retrieved 16 July 2016.
- ^ Phase Ib/II Trial of BEZ235 With Paclitaxel in Patients With HER2 Negative, Locally Advanced or Metastatic Breast Cancer
- ^ BEZ235 Phase II Trial in Patients With Advanced Pancreatic Neuroendocrine Tumors (pNET) After Failure of mTOR Inhibitor Therapy.
- ^ Pongas, G.; Fojo, T. (2016). "BEZ235: When Promising Science Meets Clinical Reality". The Oncologist. 21 (9): 1033–1034. doi:10.1634/theoncologist.2016-0243. PMC 5016067. PMID 27566248.
- ^ "A Phase Ib Study of the Dual PI3K/mTOR Inhibitor Dactolisib(BEZ235) Combined with Everolimus in Patients with AdvancedSolid Malignancies". Target Oncology. Retrieved 29 March 2017.
- ^ "A Phase I, Dose-finding Study of BEZ235 in Adult Patients With Relapsed or Refractory Acute Leukemia". clinicatrials.gov. Retrieved 29 July 2020.
- ^ Zhavoronkov A (2020). "Geroprotective and senoremediative strategies to reduce the comorbidity, infection rates, severity, and lethality in gerophilic and gerolavic infections". Aging. 12 (8): 6492–6510. doi:10.18632/aging.102988. PMC 7202545. PMID 32229705.