Etravirine
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Intelence |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Hepatic (CYP3A4, CYP2C9 & CYP2C19-mediated) |
| Elimination half-life | 41±20 hours |
| Excretion | Faeces (93.7%), urine (1.2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.546 |
| Chemical and physical data | |
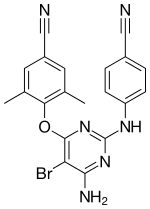
| Formula | C20H15BrN6O |
| Molar mass | 435.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Etravirine (ETR,[1] brand name Intelence, formerly known as TMC125) is a drug used for the treatment of HIV. Etravirine is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Unlike the currently available agents in the class, resistance to other NNRTIs does not seem to confer resistance to etravirine.[2] Etravirine is marketed by Janssen, a subsidiary of Johnson & Johnson. In January 2008, the Food and Drug Administration approved its use for patients with established resistance to other drugs, making it the 30th anti-HIV drug approved in the United States and the first to be approved in 2008.[3] It was also approved for use in Canada on April 1, 2008.[4]
Etravirine is licensed in the United States, Canada, Israel, Russia, Australia, New Zealand and the European Union,[5] and is under regulatory review in Switzerland.[6]
Indications and dosage[edit]
Etravirine, in combination with other anti-retrovirals, is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in antiretroviral treatment-experienced adult patients, who have evidence of viral replication and HIV-1 strains resistant to a non-nucleoside reverse transcriptase inhibitor (NNRTI) and other antiretroviral agents.
The recommended dose of etravirine is 200 mg (2 x 100 mg tablets, or 1 x 200 mg tablet as of 03/18/2011) taken twice daily following a meal. The type of food does not affect the exposure to etravirine.[7]
Contraindication[edit]
Each 100 mg etravirine tablet contains 160 mg of lactose. Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.[8]
Mechanism of action[edit]
Etravirine is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI), designed to be active against HIV with mutations that confer resistance to the two most commonly prescribed first-generation NNRTIs, mutation K103N for efavirenz and Y181C for nevirapine.[9] This potency appears to be related to etravirine's flexibility as a molecule. Etravirine is a diarylpyrimidine (DAPY), a type of organic molecule with some conformational isomerism that can bind the enzyme reverse transcriptase in multiple conformations, allowing for a more robust interaction between etravirine and the enzyme, even in the presence of mutations.[10] Other diarylpyrimidine-analogues are currently being used as anti-HIV agents, notably rilpivirine.
Warnings and risks[edit]
In 2009, the prescribing information for etravirine was modified to include "postmarketing reports of cases of Stevens–Johnson syndrome, toxic epidermal necrolysis and erythema multiforme, as well as hypersensitivity reactions characterized by rash, constitutional findings, and sometimes organ dysfunction, including liver failure. Intelence therapy should be immediately discontinued when signs and symptoms of severe skin or hypersensitivity reactions develop."[11]
Repositioning[edit]
Etravine has been studied for use in a drug repositioning application. In a paper[12] published in the medical journal Movement Disorders, etravirine was shown to cause an increase in frataxin production. Frataxin deficiency is a key component to Friedreich's ataxia, a genetically inherited disease that causes the progressive loss of coordination and muscle strength leading to motor incapacitation and the full-time use of a wheelchair.
Chemistry[edit]
Etravine forms as colourless orthorhombic crystals in space group Pna21.[13] The structures of these and of a number of solvate and salt forms have been reported.[13][14]
References[edit]
- ^ "Appendix A: Key to Acronyms". Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services. Archived from the original on 2012-08-31.
- ^ Stellbrink HJ (October 2007). "Antiviral drugs in the treatment of AIDS: what is in the pipeline ?". European Journal of Medical Research. 12 (9): 483–495. PMID 17933730.
- ^ "FDA Approves HIV Drug Etravirine". Associated Press. January 18, 2008.[dead link]
- ^ "First New NNRTI in Nearly a Decade to Benefit Canadians with HIV/AIDS" (PDF) (Press release). Janssen-Ortho Inc. 2008-04-01. Archived from the original (PDF) on 2010-11-02. Retrieved 2008-07-09.
- ^ "Intelence receives marketing authorisation in the European Union for HIV combination therapy". Tibotec. Archived from the original on 2011-09-28. Retrieved 2008-08-29.
- ^ "Etravirine (TMC125, Intelence) granted accelerated approval in US". aidsmap. Archived from the original on 2010-01-02. Retrieved 2008-01-24.
- ^ "Intelence prescribing information" (PDF). FDA. Retrieved January 19, 2012.
- ^ "Etravine: Summary of product characteristics" (PDF). EMEA. p. 5. Archived from the original (PDF) on August 20, 2016. Retrieved July 13, 2011.
- ^ Evans D (2008-01-15). "Etravirine—Countdown to Launch". AIDSmeds.com. Archived from the original on 2008-01-19. Retrieved 2008-02-02.
- ^ Das K, Clark AD, Lewi PJ, Heeres J, De Jonge MR, Koymans LM, et al. (May 2004). "Roles of conformational and positional adaptability in structure-based design of TMC125-R165335 (etravirine) and related non-nucleoside reverse transcriptase inhibitors that are highly potent and effective against wild-type and drug-resistant HIV-1 variants". Journal of Medicinal Chemistry. 47 (10): 2550–2560. doi:10.1021/jm030558s. PMID 15115397.
- ^ "FDA Medwatch Safety Information". Food and Drug Administration. Retrieved 2009-08-27.
- ^ Alfedi G, Luffarelli R, Condò I, Pedini G, Mannucci L, Massaro DS, et al. (March 2019). "Drug repositioning screening identifies etravirine as a potential therapeutic for friedreich's ataxia". Movement Disorders. 34 (3): 323–334. doi:10.1002/mds.27604. PMID 30624801. S2CID 58567610.
- ^ a b Rajput L, Sanphui P, Desiraju GR (2013-08-07). "New Solid Forms of the Anti-HIV Drug Etravirine: Salts, Cocrystals, and Solubility". Crystal Growth & Design. 13 (8): 3681–3690. doi:10.1021/cg4007058. ISSN 1528-7483.
- ^ Muresan-Pop M, Macavei S, Turza A, Borodi G (November 2021). "New solvates and a salt of the anti-HIV compound etravirine". Acta Crystallographica Section C: Structural Chemistry. 77 (Pt 11): 698–706. doi:10.1107/S2053229621010482. PMID 34738540. S2CID 243761396.
External links[edit]
- "Etravirine". Drug Information Portal. U.S. National Library of Medicine.