Protein acetylation
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
Protein acetylation (and deacetylation) are acetylation reactions that occur within living cells as drug metabolism, by enzymes in the liver and other organs (e. g., the brain). Pharmaceuticals frequently employ acetylation to enable such esters to cross the blood–brain barrier (and placenta), where they are deacetylated by enzymes (carboxylesterases) in a manner similar to acetylcholine. Examples of acetylated pharmaceuticals are diacetylmorphine (heroin), acetylsalicylic acid (aspirin), THC-O-acetate, and diacerein. Conversely, drugs such as isoniazid are acetylated within the liver during drug metabolism. A drug that depends on such metabolic transformations in order to act is termed a prodrug.
Acetylation is an important modification of proteins in cell biology; and proteomics studies have identified thousands of acetylated mammalian proteins.[1][2][3] Acetylation occurs as a co-translational and post-translational modification of proteins, for example, histones, p53, and tubulins. Among these proteins, chromatin proteins and metabolic enzymes are highly represented, indicating that acetylation has a considerable impact on gene expression and metabolism. In bacteria, 90% of proteins involved in central metabolism of Salmonella enterica are acetylated.[4][5]
N-terminal acetylation[edit]

N-terminal acetylation is one of the most common co-translational covalent modifications of proteins in eukaryotes, and it is crucial for the regulation and function of different proteins. N-terminal acetylation plays an important role in the synthesis, stability and localization of proteins. About 85% of all human proteins and 68% in yeast are acetylated at their Nα-terminus.[6] Several proteins from prokaryotes and archaea are also modified by N-terminal acetylation.
N-terminal Acetylation is catalyzed by a set of enzyme complexes, the N-terminal acetyltransferases (NATs). NATs transfer an acetyl group from acetyl-coenzyme A (Ac-CoA) to the α-amino group of the first amino acid residue of the protein. Different NATs are responsible for the acetylation of nascent protein N-terminal, and the acetylation was found to be irreversible so far.[7]
N-terminal acetyltransferases[edit]
To date, seven different NATs have been found in humans - NatA, NatB, NatC, NatD, NatE, NatF and NatH. Each of these different enzyme complexes is specific for different amino acids or amino acid sequences which is shown in the following table.
Table 1. The Composition and Substrate specificity of NATs.
| NAT | Subunits (catalytic subunits are in bold.) | Substrates |
|---|---|---|
| NatA | Naa10 (Ard1) Naa15 (Nat1) | Ser-, Ala-, Gly-, Thr-, Val-, Cys- N-termini |
| NatB | Naa20 (Nat3) Naa25 (Mdm20) | Met-Glu-, Met-Asp-, Met-Asn-, Met-Gln- N-termini |
| NatC | Naa30 (Mak3) Naa35 (Mak10) Naa38 (Mak31) | Met-Leu-, Met-Ile-, Met-Trp-, Met-Phe- N-termini |
| NatD | Naa40 (Nat4) | Ser-Gly-Gly-, Ser-Gly-Arg- N-termini |
| NatE | Naa50 (Nat5) Naa10 (Ard1) Naa15 (Nat1) | Met-Leu-, Met-Ala-, Met-Lys-, Met-Met- N-termini |
| NatF | Naa60 | Met-Lys-, Met-Leu-, Met-Ile-, Met-Trp-, Met-Phe- N-termini |
| NatH | Naa80 | Actin- N-termini |
NatA[edit]

NatA is composed of two subunits, the catalytic subunit Naa10 and the auxiliary subunit Naa15. NatA subunits are more complex in higher eukaryotes than in lower eukaryotes. In addition to the genes NAA10 and NAA15, the mammal-specific genes NAA11 and NAA16, make functional gene products, which form different active NatA complexes. Four possible hNatA catalytic-auxiliary dimers are formed by these four proteins. However, Naa10/Naa15 is the most abundant NatA.[9]
NatA acetylates Ser, Ala-, Gly-, Thr-, Val- and Cys N-termini after the initiator methionine is removed by methionine amino-peptidases. These amino acids are more frequently expressed in the N-terminal of proteins in eukaryotes, so NatA is the major NAT corresponding to the whole number of its potential substrates.[10]
Several different interaction partners are involved in the N-terminal acetylation by NatA. Huntingtin interacting protein K (HYPK) interacts with hNatA on the ribosome to affect the N-terminal acetylation of a subset of NatA substrates. Subunits hNaa10 and hNaa15 will increase the tendency for aggregation of Huntingtin if HYPK is depleted. Hypoxia-inducible factor (HIF)-1α has also been found to interact with hNaa10 to inhibit hNaa10-mediated activation of β-catenin transcriptional activity.[11]
NatB[edit]
NatB complexes are composed of the catalytic subunit Naa20p and the auxiliary subunit Naa25p, which are both found in yeast and humans. In yeast, all the NatB subunits are ribosome-associated; but in humans, NatB subunits are both found to be ribosome-associated and non-ribosomal form. NatB acetylates the N-terminal methionine of substrates starting with Met-Glu-, Met-Asp-, Met-Asn- or Met-Gln- N termini.
NatC[edit]
NatC complex consists of one catalytic subunit Naa30p and two auxiliary subunits Naa35p and Naa38p. All three subunits are found on the ribosome in yeast, but they are also found in non-ribosomal NAT forms like Nat2. NatC complex acetylates the N-terminal methionine of substrates Met-Leu-, Met-Ile-, Met-Trp- or Met-Phe N-termini.
NatD[edit]
NatD is only composed with the catalytic unit Naa40p and Naa40p and it is conceptually different form the other NATs. At first, only two substrates, H2A and H4 have been identified in yeast and humans. Secondly, the substrate specificity of Naa40p lies within the first 30-50 residues which are quite larger than the substrate specificity of other NATs. The acetylation of histones by NatD is partially associate with ribosomes and the amino acids substrates are the very N-terminal residues, which makes it different from lysine N-acetyltransferases (KATs).[12]
NatE[edit]
NatE complex consists with subunit Naa50p and two NatA subunits, Naa10p and Naa15p. The N terminus of Naa50p substrates is different from those acetylated by the NatA activity of Naa10p.[13] NAA50 in plants is essential to control plant growth, development, and stress responses and NAA50 function is highly conserved between humans and plants.[14][15][16][17]
NatF[edit]

NatF is a NAT that is composed of the Naa60 enzyme. Initially, it was thought that NatF was only found in higher eukaryotes, since it was absent from yeast.[18] However, it was later found that Naa60 is found throughout the eukaryotic domain, but was secondarily lost in the fungi lineage.[19] Compared to yeast, NatF contributes to the higher abundance of N-terminal acetylation in humans. NatF complex acetylates the N-terminal methionine of substrates Met-Lys-, Met-Leu-, Met-Ile-, Met-Trp- and Met-Phe N termini which are partly overlapping with NatC and NatE.[6] NatF has been shown to have an organellar localization and acetylates cytosolic N-termini of transmembrane proteins.[20] The organellar localization of Naa60 is mediated by its unique C-terminus, which consists of two alpha helices that peripherally associate with the membrane and mediate interactions with PI(4)P.[21]
NAA80/NatH[edit]
NAA80/NatH is an N-terminal acetyltransferase that specifically acetylates the N-terminus of actin.[22]
N-terminal acetylation function[edit]
Protein stability[edit]
N-terminal acetylation of proteins can affect protein stability, but the results and mechanism were not very clear until now.[23] It was believed that N-terminal acetylation protects proteins from being degraded as Nα-acetylation N-termini were supposed to block N-terminal ubiquitination and subsequent protein degradation.[24] However, several studies have shown that the N-terminal acetylated protein have a similar degradation rate as proteins with a non-blocked N-terminus.[25]
Protein localization[edit]
N-terminal acetylation has been shown that it can steer the localization of proteins. Arl3p is one of the ‘Arf-like’ (Arl) GTPases, which is crucial for the organization of membrane traffic.[26] It requires its Nα-acetyl group for its targeting to the Golgi membrane by the interaction with Golgi membrane-residing protein Sys1p. If the Phe or Tyr is replaced by an Ala at the N-terminal of Arl3p, it can no longer localized to the Golgi membrane, indicating that Arl3p needs its natural N-terminal residues which could be acetylated for proper localization.[27]
Metabolism and apoptosis[edit]
Protein N-terminal acetylation has also been proved to relate with cell cycle regulation and apoptosis with protein knockdown experiments. Knockdown of the NatA or the NatC complex leads to the induction of p53-dependent apoptosis, which may indicate that the anti-apoptotic proteins were less or no longer functional because of reduced protein N-terminal acetylation.[28] But in contrast, the caspase-2, which is acetylated by NatA, can interact with the adaptor protein RIP associated Ich-1/Ced-3 homologous protein with a death domain (RAIDD). This could activate caspase-2 and induce cell apoptosis.[29]
Protein synthesis[edit]
Ribosome proteins play an important role in the protein synthesis, which could also be N-terminal acetylated. The N-terminal acetylation of the ribosome proteins may have an effect on protein synthesis. A decrease of 27% and 23% in the protein synthesis rate was observed with NatA and NatB deletion strains. A reduction of translation fidelity was observed in the NatA deletion strain and a defect in ribosome was noticed in the NatB deletion strain.[30]
Cancer[edit]
NATs have been suggested to act as both onco-proteins and tumor suppressors in human cancers, and NAT expression may be increased and decreased in cancer cells. Ectopic expression of hNaa10p increased cell proliferation and up regulation of gene involved in cell survival proliferation and metabolism. Overexpression of hNaa10p was in the urinary bladder cancer, breast cancer and cervical carcinoma.[31] But a high level expression of hNaa10p could also suppress tumor growth and a reduced level of expressed hNaa10p is associated with a poor prognosis, large tumors and more lymph node metastases.
Table 2. Overview of the expression of NatA subunits in various cancer tissues[32]
| Nat subunits | Cancer tissue | Expression pattern |
|---|---|---|
| hNaa10 | lung cancer, breast cancer, colorectal cancer, hepatocellular carcinoma | high in tumors |
| hNaa10 | lung cancer, breast cancer, pancreatic cancer, ovarian cancer | loss of heterozygosity in tumors |
| hNaa10 | breast cancer, gastric cancer, lung cancer | high in primary tumors, but low with lymph node metastases |
| hNaa10 | Non-small cell lung cancer | low in tumors |
| hNaa15 | papillary thyroid carcinoma, gastric cancer | high in tumors |
| hNaa15 | neuroblastoma | high in advanced stage tumors |
| hNaa11 | hepatocellular carcinoma | loss of heterozygosity in tumors |
Lysine acetylation and deacetylation[edit]

Proteins are typically acetylated on lysine residues and this reaction relies on acetyl-coenzyme A as the acetyl group donor. In histone acetylation and deacetylation, histone proteins are acetylated and deacetylated on lysine residues in the N-terminal tail as part of gene regulation. Typically, these reactions are catalyzed by enzymes with histone acetyltransferase (HAT) or histone deacetylase (HDAC) activity, although HATs and HDACs can modify the acetylation status of non-histone proteins as well.[33]
The regulation of transcription factors, effector proteins, molecular chaperones, and cytoskeletal proteins by acetylation and deacetylation is a significant post-translational regulatory mechanism[34] These regulatory mechanisms are analogous to phosphorylation and dephosphorylation by the action of kinases and phosphatases. Not only can the acetylation state of a protein modify its activity but there has been recent suggestion that this post-translational modification may also crosstalk with phosphorylation, methylation, ubiquitination, sumoylation, and others for dynamic control of cellular signaling.[35] The regulation of tubulin protein is an example of this in mouse neurons and astroglia.[36][37] A tubulin acetyltransferase is located in the axoneme, and acetylates the α-tubulin subunit in an assembled microtubule. Once disassembled, this acetylation is removed by another specific deacetylase in the cell cytosol. Thus axonemal microtubules, which have a long half-life, carry a "signature acetylation," which is absent from cytosolic microtubules that have a shorter half-life.
In the field of epigenetics, histone acetylation (and deacetylation) have been shown to be important mechanisms in the regulation of gene transcription. Histones, however, are not the only proteins regulated by posttranslational acetylation. The following are examples of various other proteins with roles in regulating signal transduction, whose activities are also affected by acetylation and deacetylation.
p53[edit]
The p53 protein is a tumor suppressor that plays an important role in the signal transactions in cells, especially in maintaining the stability of the genome by preventing mutation. Therefore, it is also known as “the guardian of the genome". It also regulates the cell cycle and arrests cell growth by activating a regulator of the cell cycle, p21. Under severe DNA damage, it also initiates programmed cell death.The function of p53 is negatively regulated by oncoprotein Mdm2. Studies suggested that Mdm2 will form a complex with p53 and prevent it from binding to specific p53-responsive genes.[38][39]
Acetylation of p53[edit]

The acetylation of p53 is indispensable for its activation. It has been reported that the acetylation level of p53 will increase significantly when the cell undergoes stress. Acetylation sites have been observed on the DNA binding domain (K164 and K120) and the C terminus.[40] Acetylation sites demonstrate significant redundancy: if only one acetylation site is inactivated by mutation to arginine, the expression of p21 is still observed. However, if multiple acetylation sites are blocked, the expression of p21 and the suppression of cell growth caused by p53 is completely lost. In addition, the acetylation of p53 prevents its binding to the repressor Mdm2 on DNA.[41] In addition, it is suggested that the p53 acetylation is crucial for its transcription-independent proapoptotic functions.[42] An acetylation site of the C-terminus was investigated by molecular dynamics simulations and circular dichroism spectroscopy, and it was suggested that the acetylation changes the structural ensemble of the C-terminus.[43]
Implications for cancer therapy[edit]
Since the major function of p53 is tumor suppressor, the idea that activation of p53 is an appealing strategy for cancer treatment. Nutlin-3[44] is a small molecule designed to target p53 and Mdm2 interaction that kept p53 from deactivation.[45] Reports also shown that the cancer cell under the Nutilin-3a treatment, acetylation of lys 382 was observed in the c-terminal of p53.[46][47]
Microtubule[edit]

The structure of microtubules is long, hollow cylinder dynamically assembled from α/β-tubulin dimers. They play an essential role in maintaining the structure of the cell as well as cell processes, for example, movement of organelles.[48] In addition, microtubule is responsible of forming mitotic spindle in eukaryotic cells to transport chromosomes in cell division.[49][50]
Acetylation of tubulin[edit]
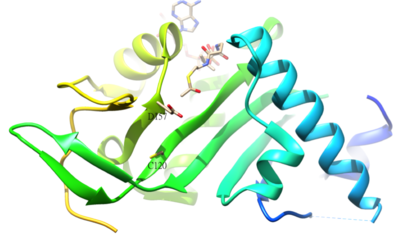
The acetylated residue of α-tubulin is K40, which is catalyzed by α-tubulin acetyl-transferase (α-TAT) in human. The acetylation of K40 on α-tubulin is a hallmark of stable microtubules. The active site residues D157 and C120 of α-TAT1 are responsible for the catalysis because of the shape complementary to α-Tubulin. In addition, some unique structural features such as β4-β5 hairpin, C-terminal loop, and α1-α2 loop regions are important for specific α-Tubulin molecular recognition.[51] The reverse reaction of the acetylation is catalyzed by histone deacetylase 6.[52]
Implications for cancer therapy[edit]
Since microtubules play an important role in cell division, especially in the G2/M phase of the cell cycle, attempts have been made to impede microtubule function using small molecule inhibitors, which have been successfully used in clinics as cancer therapies.[53] For example, the vinca alkaloids and taxanes selectively bind and inhibit microtubules, leading to cell cycle arrest.[54] The identification of the crystal structure of acetylation of α-tubulin acetyl-transferase (α-TAT) also sheds a light on the discovery of small molecule that could modulate the stability or de-polymerization of tubulin. In other words, by targeting α-TAT, it is possible to prevent the tubulin from acetylation and result in the destabilization of tubulin, which is a similar mechanism for tubulin destabilizing agents.[51]
STAT3[edit]
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor that is phosphorylated by receptor associated kinases, for example, Janus-family tyrosine kinases, and translocate to nucleus. STAT3 regulates several genes in response to growth factors and cytokines and play an important role in cell growth. Therefore, STAT3 facilitates oncogenesis in a variety of cell growth related pathways. On the other hand, it also play a role in the tumor suppressor.[55]
Acetylation of STAT3[edit]

The acetylation of Lys685 of STAT3 is important for STAT3 homo-dimerization, which is essential for the DNA-binding and the transcriptional activation of oncogenes. The acetylation of STAT3 is catalyzed by histone acetyltransferase p300, and reversed by type 1 histone deacetylase. The lysine acetylation of STAT3 is also elevated in cancer cells.[56]
Therapeutic implications for cancer therapy[edit]
Since the acetylation of STAT3 is important for its oncogenic activity and the fact that the level of acetylated STAT3 is high in cancer cells, it is implied that targeting acetylated STAT3 for chemoprevention and chemotherapy is a promising strategy. This strategy is supported by treating resveratrol, an inhibitor of acetylation of STAT3, in cancer cell line reverses aberrant CpG island methylation.[57]
See also[edit]
References[edit]
- ^ Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M (2009). "Lysine acetylation targets protein complexes and co-regulates major cellular functions". Science. 325 (5942): 834–840. Bibcode:2009Sci...325..834C. doi:10.1126/science.1175371. PMID 19608861. S2CID 206520776.
- ^ Fritz KS, Galligan JJ, Hirschey MD, Verdin E, Petersen DR (2012). "Mitochondrial acetylome analysis in a mouse model of alcohol-induced liver injury utilizing SIRT3 knockout mice". J. Proteome Res. 11 (3): 1633–1643. doi:10.1021/pr2008384. PMC 3324946. PMID 22309199.
- ^ Brook T. "Protein Acetylation: Much More than Histone Acetylation". Cayman Chemical. Archived from the original on 2014-02-28.
- ^ Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL (2010). "Regulation of cellular metabolism by protein lysine acetylation". Science. 327 (5968): 1000–1004. Bibcode:2010Sci...327.1000Z. doi:10.1126/science.1179689. PMC 3232675. PMID 20167786.
- ^ Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, et al. (February 2010). "Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux". Science. 327 (5968): 1004–7. Bibcode:2010Sci...327.1004W. doi:10.1126/science.1179687. PMC 4183141. PMID 20167787.
- ^ a b Van Damme P, Hole K, Pimenta-Marques A, Helsens K, Vandekerckhove J, Martinho RG, Gevaert K, Arnesen T (2011). "NatF contributes to an evolutionary shift in protein N-terminal acetylation and is important for normal chromosome segregation". PLOS Genet. 7 (7): e1002169. doi:10.1371/journal.pgen.1002169. PMC 3131286. PMID 21750686.
- ^ Starheim KK, Gevaert K, Arnesen T (2012). "Protein N-terminal acetyltransferases: when the start matters". Trends Biochem. Sci. 37 (4): 152–161. doi:10.1016/j.tibs.2012.02.003. PMID 22405572.
- ^ Liszczak G, Goldberg JM, Foyn H, Petersson EJ, Arnesen T, Marmorstein R (2013). "Molecular basis for N-terminal acetylation by the heterodimeric NatA complex". Nat. Struct. Mol. Biol. 20 (9): 1098–105. doi:10.1038/nsmb.2636. PMC 3766382. PMID 23912279.
- ^ Starheim KK, Gromyko D, Velde R, Varhaug JE, Arnesen T (2009). "Composition and biological significance of the human Nalpha-terminal acetyltransferases". BMC Proceedings. 3 (Suppl 6): S3. doi:10.1186/1753-6561-3-s6-s3. PMC 2722096. PMID 19660096.
- ^ Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K (2009). "Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans". Proc. Natl. Acad. Sci. U.S.A. 106 (20): 8157–8162. Bibcode:2009PNAS..106.8157A. doi:10.1073/pnas.0901931106. PMC 2688859. PMID 19420222.
- ^ Arnesen T, Starheim KK, Van Damme P, Evjenth R, Dinh H, Betts MJ, Ryningen A, Vandekerckhove J, Gevaert K, Anderson D (2010). "The chaperone-like protein HYPK acts together with NatA in cotranslational N-terminal acetylation and prevention of Huntingtin aggregation". Mol. Cell. Biol. 30 (8): 1898–1909. doi:10.1128/mcb.01199-09. PMC 2849469. PMID 20154145.
- ^ Hole K, Van Damme P, Dalva M, Aksnes H, Glomnes N, Varhaug JE, Lillehaug JR, Gevaert K, Arnesen T (2011). "The human N-alpha-acetyltransferase 40 (hNaa40p/hNatD) is conserved from yeast and N-terminally acetylates histones H2A and H4". PLOS ONE. 6 (9): e24713. Bibcode:2011PLoSO...624713H. doi:10.1371/journal.pone.0024713. PMC 3174195. PMID 21935442.
- ^ Gautschi M, Just S, Mun A, Ross S, Rücknagel P, Dubaquié Y, et al. (October 2003). "The yeast N(alpha)-acetyltransferase NatA is quantitatively anchored to the ribosome and interacts with nascent polypeptides". Molecular and Cellular Biology. 23 (20): 7403–14. doi:10.1128/mcb.23.20.7403-7414.2003. PMC 230319. PMID 14517307.
- ^ Hartman S (August 2020). "The Meaning of an End: N-Terminal Acetyltransferase NAA50 Controls Plant Growth and Stress Responses". Plant Physiology. 183 (4): 1410–1411. doi:10.1104/pp.20.00794. PMC 7401126. PMID 32747486.
- ^ Armbruster L, Linster E, Boyer JB, Brünje A, Eirich J, Stephan I, et al. (August 2020). "NAA50 Is an Enzymatically Active Nα-Acetyltransferase That Is Crucial for Development and Regulation of Stress Responses". Plant Physiology. 183 (4): 1502–1516. doi:10.1104/pp.20.00222. PMC 7401105. PMID 32461302.
- ^ Neubauer M, Innes RW (August 2020). "Loss of the Acetyltransferase NAA50 Induces Endoplasmic Reticulum Stress and Immune Responses and Suppresses Growth". Plant Physiology. 183 (4): 1838–1854. doi:10.1104/pp.20.00225. PMC 7401112. PMID 32457093.
- ^ Feng J, Hu J, Li Y, Li R, Yu H, Ma L (September 2020). "The N-Terminal Acetyltransferase Naa50 Regulates Arabidopsis Growth and Osmotic Stress Response". Plant & Cell Physiology. 61 (9): 1565–1575. doi:10.1093/pcp/pcaa081. PMID 32544241.
- ^ Van Damme P, Hole K, Pimenta-Marques A, Helsens K, Vandekerckhove J, Martinho RG, et al. (July 2011). "NatF contributes to an evolutionary shift in protein N-terminal acetylation and is important for normal chromosome segregation". PLOS Genetics. 7 (7): e1002169. doi:10.1371/journal.pgen.1002169. PMC 3131286. PMID 21750686.
- ^ Rathore OS, Faustino A, Prudêncio P, Van Damme P, Cox CJ, Martinho RG (February 2016). "Absence of N-terminal acetyltransferase diversification during evolution of eukaryotic organisms". Scientific Reports. 6: 21304. Bibcode:2016NatSR...621304R. doi:10.1038/srep21304. PMC 4748286. PMID 26861501.
- ^ Aksnes H, Van Damme P, Goris M, Starheim KK, Marie M, Støve SI, et al. (March 2015). "An organellar nα-acetyltransferase, naa60, acetylates cytosolic N termini of transmembrane proteins and maintains Golgi integrity". Cell Reports. 10 (8): 1362–74. doi:10.1016/j.celrep.2015.01.053. hdl:1956/10959. PMID 25732826.
- ^ Aksnes H, Goris M, Strømland Ø, Drazic A, Waheed Q, Reuter N, Arnesen T (April 2017). "Molecular determinants of the N-terminal acetyltransferase Naa60 anchoring to the Golgi membrane". The Journal of Biological Chemistry. 292 (16): 6821–6837. doi:10.1074/jbc.M116.770362. PMC 5399128. PMID 28196861.
- ^ Drazic A, Aksnes H, Marie M, Boczkowska M, Varland S, Timmerman E, Foyn H, Glomnes N, Rebowski G, Impens F, Gevaert K, Dominguez R, and Arnesen T (2018). "NAA80 is actin's N-terminal acetyltransferase and regulates cytoskeleton assembly and cell motility". Proc Natl Acad Sci U S A. 115 (17): 4399–4404. doi:10.1073/pnas.1718336115. PMC 5924898. PMID 29581253.
- ^ Hollebeke J, Van Damme P, Gevaert K (2012). "N-terminal acetylation and other functions of Nα-acetyltransferases". Biol. Chem. 393 (4): 291–8. doi:10.1515/hsz-2011-0228. PMID 22718636. S2CID 40566358.
- ^ Hershko A, Heller H, Eytan E, Kaklij G, Rose IA (1984). "Role of the alpha-amino group of protein in ubiquitin-mediated protein breakdown". Proc. Natl. Acad. Sci. U.S.A. 81 (22): 7021–5. Bibcode:1984PNAS...81.7021H. doi:10.1073/pnas.81.22.7021. PMC 392068. PMID 6095265.
- ^ Hwang CS, Shemorry A, Varshavsky A (2010). "N-terminal acetylation of cellular proteins creates specific degradation signals". Science. 327 (5968): 973–977. Bibcode:2010Sci...327..973H. doi:10.1126/science.1183147. PMC 4259118. PMID 20110468.
- ^ Behnia R, Panic B, Whyte JR, Munro S (2004). "Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p". Nat. Cell Biol. 6 (5): 405–413. doi:10.1038/ncb1120. PMID 15077113. S2CID 22954283.
- ^ Starheim KK, Gromyko D, Evjenth R, Ryningen A, Varhaug JE, Lillehaug JR, Arnesen T (2009). "Knockdown of human N alpha-terminal acetyltransferase complex C leads to p53-dependent apoptosis and aberrant human Arl8b localization". Mol. Cell. Biol. 29 (13): 3569–3581. doi:10.1128/mcb.01909-08. PMC 2698767. PMID 19398576.
- ^ Gromyko D, Arnesen T, Ryningen A, Varhaug JE, Lillehaug JR (2010). "Depletion of the human Nα-terminal acetyltransferase A induces p53-dependent apoptosis and p53-independent growth inhibition". Int. J. Cancer. 127 (12): 2777–2789. doi:10.1002/ijc.25275. PMID 21351257.
- ^ Yi CH, Pan H, Seebacher J, Jang IH, Hyberts SG, Heffron GJ, Vander Heiden MG, Yang R, Li F, Locasale JW, Sharfi H, Zhai B, Rodriguez-Mias R, Luithardt H, Cantley LC, Daley GQ, Asara JM, Gygi SP, Wagner G, Liu CF, Yuan J (2011). "Metabolic regulation of protein N-alpha-acetylation by Bcl-xL promotes cell survival". Cell. 146 (4): 607–620. doi:10.1016/j.cell.2011.06.050. PMC 3182480. PMID 21854985.
- ^ Kamita M, Kimura Y, Ino Y, Kamp RM, Polevoda B, Sherman F, Hirano H (2011). "N(α)-Acetylation of yeast ribosomal proteins and its effect on protein synthesis". J Proteomics. 74 (4): 431–441. doi:10.1016/j.jprot.2010.12.007. PMID 21184851.
- ^ Yu M, Gong J, Ma M, Yang H, Lai J, Wu H, Li L, Li L, Tan D (2009). "Immunohistochemical analysis of human arrest-defective-1 expressed in cancers in vivo". Oncol. Rep. 21 (4): 909–15. doi:10.3892/or_00000303. PMID 19287988.
- ^ Kalvik TV, Arnesen T (2013). "Protein N-terminal acetyltransferases in cancer". Oncogene. 32 (3): 269–276. doi:10.1038/onc.2012.82. PMID 22391571.
- ^ Sadoul K, Boyault C, Pabion M, Khochbin S (2008). "Regulation of protein turnover by acetyltransferases and deacetylases". Biochimie. 90 (2): 306–12. doi:10.1016/j.biochi.2007.06.009. PMID 17681659.
- ^ Glozak MA, Sengupta N, Zhang X, Seto E (2005). "Acetylation and deacetylation of non-histone proteins". Gene. 363: 15–23. doi:10.1016/j.gene.2005.09.010. PMID 16289629.
- ^ Yang XJ, Seto E (2008). "Lysine acetylation: codified crosstalk with other posttranslational modifications". Mol. Cell. 31 (4): 449–61. doi:10.1016/j.molcel.2008.07.002. PMC 2551738. PMID 18722172.
- ^ Eddé B, Denoulet P, de Néchaud B, Koulakoff A, Berwald-Netter Y, Gros F (1989). "Posttranslational modifications of tubulin in cultured mouse brain neurons and astroglia". Biol. Cell. 65 (2): 109–117. doi:10.1016/0248-4900(89)90018-x. PMID 2736326.
- ^ Maruta H, Greer K, Rosenbaum JL (1986). "The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules". J. Cell Biol. 103 (2): 571–579. doi:10.1083/jcb.103.2.571. PMC 2113826. PMID 3733880.
- ^ Alberts B (March 2002). Molecular Biology of the Cell. Garland Science. ISBN 0815332181.
- ^ Weinberg RA (2013). Biology of cancer (2. ed.). [S.l.]: Garland Science. ISBN 978-0815342205.
- ^ Brooks CL, Gu W (2011). "The impact of acetylation and deacetylation on the p53 pathway". Protein Cell. 2 (6): 456–462. doi:10.1007/s13238-011-1063-9. PMC 3690542. PMID 21748595.
- ^ Tang Y, Zhao W, Chen Y, Zhao Y, Gu W (2008). "Acetylation is indispensable for p53 activation". Cell. 133 (4): 612–626. doi:10.1016/j.cell.2008.03.025. PMC 2914560. PMID 18485870.
- ^ Yamaguchi H, Woods NT, Piluso LG, Lee HH, Chen J, Bhalla KN, Monteiro A, Liu X, Hung MC, Wang HG (2009). "p53 acetylation is crucial for its transcription-independent proapoptotic functions". J. Biol. Chem. 284 (17): 11171–11183. doi:10.1074/jbc.M809268200. PMC 2670122. PMID 19265193.
- ^ Iida S, Mashimo T, Kurosawa T, Hojo H, Muta H, Goto Y, et al. (December 2016). "Variation of free-energy landscape of the p53 C-terminal domain induced by acetylation: Enhanced conformational sampling". Journal of Computational Chemistry. 37 (31): 2687–2700. doi:10.1002/jcc.24494. PMC 5242334. PMID 27735058.
- ^ Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA (2004). "In vivo activation of the p53 pathway by small-molecule antagonists of MDM2". Science. 303 (5659): 844–848. Bibcode:2004Sci...303..844V. doi:10.1126/science.1092472. PMID 14704432. S2CID 16132757.
- ^ Shangary S, Wang S (2009). "Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy". Annu. Rev. Pharmacol. Toxicol. 49 (1): 223–241. doi:10.1146/annurev.pharmtox.48.113006.094723. PMC 2676449. PMID 18834305.
- ^ Zajkowicz A, Krześniak M, Matuszczyk I, Głowala-Kosińska M, Butkiewicz D, Rusin M (2013). "Nutlin-3a, an MDM2 antagonist and p53 activator, helps to preserve the replicative potential of cancer cells treated with a genotoxic dose of resveratrol". Mol. Biol. Rep. 40 (8): 5013–5026. doi:10.1007/s11033-013-2602-7. PMC 3723979. PMID 23666059.
- ^ Kumamoto K, Spillare EA, Fujita K, Horikawa I, Yamashita T, Appella E, Nagashima M, Takenoshita S, Yokota J, Harris CC (2008). "Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence". Cancer Res. 68 (9): 3193–3203. doi:10.1158/0008-5472.CAN-07-2780. PMC 2440635. PMID 18451145.
- ^ Kreis T, Vale R, eds. (1999). Guidebook to the cytoskeletal and motor proteins (2nd ed.). Oxford [u.a.]: Oxford Univ. Press. ISBN 0198599560.
- ^ Lodish H (2013). Molecular cell biology (7th ed.). New York: W.H. Freeman and Co. ISBN 978-1429234139.
- ^ Fojo T, ed. (2008). The role of microtubules in cell biology, neurobiology, and oncology ([Online-Ausg.] ed.). Totowa, N. J.: Humana Press. ISBN 978-1588292940.
- ^ a b Friedmann DR, Aguilar A, Fan J, Nachury MV, Marmorstein R (2012). "Structure of the α-tubulin acetyltransferase, αTAT1, and implications for tubulin-specific acetylation". Proc. Natl. Acad. Sci. U.S.A. 109 (48): 19655–19660. Bibcode:2012PNAS..10919655F. doi:10.1073/pnas.1209357109. PMC 3511727. PMID 23071314.
- ^ Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (2002). "HDAC6 is a microtubule-associated deacetylase". Nature. 417 (6887): 455–458. Bibcode:2002Natur.417..455H. doi:10.1038/417455a. PMID 12024216. S2CID 4373254.
- ^ Teresa Carlomagno, ed. (2009). Tubulin-binding agents : synthetic, structural, and mechanistic insights. contributions by K.-H. Altmann. Berlin: Springer. ISBN 978-3540690368.
- ^ Zito TL, Lemke TL, Williams DA, Roche VG, William S, eds. (2013). Foye's principles of medicinal chemistry (7th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. ISBN 978-1609133450.
- ^ Müller-Decker FM, Klingmüller UK (2009). Cellular signal processing : an introduction to the molecular mechanisms of signal transduction. New York: Garland Science. ISBN 978-0815342151.
- ^ Yuan ZL, Guan YJ, Chatterjee D, Chin YE (2005). "Stat3 dimerization regulated by reversible acetylation of a single lysine residue". Science. 307 (5707): 269–273. Bibcode:2005Sci...307..269Y. doi:10.1126/science.1105166. PMID 15653507. S2CID 25269192.
- ^ Lee H, Zhang P, Herrmann A, Yang C, Xin H, Wang Z, Hoon DS, Forman SJ, Jove R, Riggs AD, Yu H (2012). "Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation". Proc. Natl. Acad. Sci. U.S.A. 109 (20): 7765–7769. Bibcode:2012PNAS..109.7765L. doi:10.1073/pnas.1205132109. PMC 3356652. PMID 22547799.