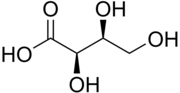
Threonic acid
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
| Names | |
|---|---|
| IUPAC name (2R,3S)-2,3,4-Trihydroxybutanoic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C4H8O5 | |
| Molar mass | 136.103 g·mol−1 |
| Conjugate base | Threonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Threonic acid is a sugar acid derived from threose. The l-isomer is a metabolite of ascorbic acid (vitamin C).[1] One study suggested that because l-threonate inhibits DKK1 expression in vitro, it may have potential in treatment of androgenic alopecia.[2]
References[edit]
- ^ S Englard and S Seifter (1986). "The Biochemical Functions of Ascorbic Acid". Annual Review of Nutrition. 6: 365–406. doi:10.1146/annurev.nu.06.070186.002053. PMID 3015170.
- ^ Kwack, M. H.; Ahn, J. S.; Kim, M. K.; Kim, J. C.; Sun, Y. K. (2010). "Preventable effect of L-threonate, an ascorbate metabolite, on androgen-driven balding via repression of dihydrotestosteroneinduced dickkopf-1 expression in human hair dermal papilla cells". BMB Reports. 43 (10): 688–692. doi:10.5483/BMBRep.2010.43.10.688. PMID 21034532.