Threose
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
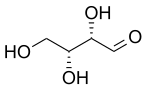 D-Threose | |
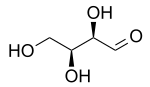 L-Threose | |
| Names | |
|---|---|
| IUPAC names D-Threose L-Threose [2] | |
| Systematic IUPAC name (2S,3R)-2,3,4-Trihydroxybutanal (D) (2R,3S)-2,3,4-Trihydroxybutanal (L) | |
| Other names Threotetrose | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.199 |
PubChem CID |
|
| UNII |
|
| |
| |
| Properties | |
| C4H8O4 | |
| Molar mass | 120.104 g·mol−1 |
| Appearance | Syrup |
| Very soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Threose is a four-carbon monosaccharide with molecular formula C4H8O4. It has a terminal aldehyde group rather than a ketone in its linear chain, and so is considered part of the aldose family of monosaccharides. The threose name can be used to refer to both the D- and L-stereoisomers, and more generally to the racemic mixture (D/L-, equal parts D- and L-) as well as to the more generic threose structure (absolute stereochemistry unspecified).
The prefix "threo" which derives from threose (and "erythro" from a corresponding diastereomer erythrose) offer a useful way to describe general organic structures with adjacent chiral centers, where "the prefixes... designate the relative configuration of the centers".[3] As is depicted in a Fischer projection of D-threose, the adjacent substituents will have a syn orientation in the isomer referred to as "threo", and are anti in the isomer referred to as "erythro".[3][4]

See also[edit]
References[edit]
- ^ Merck Index, 11th Edition, 9317
- ^ https://iupac.qmul.ac.uk/2carb
- ^ a b Formulas Using Other Configurational Notations, W. Rausch, accessed 1 March 2011
- ^ Prof. Rausch helpfully notes that the prefixes "may be applied to racemic compounds, as well as pure enantiomers and meso compounds", and that when depicted in the common "zig-zag" representation, adjacent "substituents may lie on the same side of the carbon chain... [syn] or on opposite sides... [anti]", which is opposite of their depiction in a Fischer projection.