Deoxyguanosine triphosphate
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
 | |
| Names | |
|---|---|
| IUPAC name 2′-Deoxyguanosine 5′-(tetrahydrogen triphosphate) | |
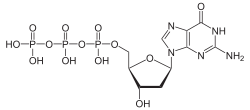
| Systematic IUPAC name O1-{[(2R,3S,5R)-5-(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3-hydroxyoxolan-2-yl]methyl} tetrahydrogen triphosphate | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.018.080 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C10H16N5O13P3 | |
| Molar mass | 507.181023 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Deoxyguanosine triphosphate[1] (dGTP) is a nucleoside triphosphate, and a nucleotide precursor used in cells for DNA synthesis. The substance is used in the polymerase chain reaction technique, in sequencing, and in cloning. It is also the competitor of inhibition onset by acyclovir in the treatment of HSV virus.[2]
References
[edit]- ^ Nelson, David L.; Cox, Michael M. (2005). Principles of Biochemistry (4th ed.). New York: W. H. Freeman. ISBN 0-7167-4339-6.
- ^ Furman PA, Lambe CU, Nelson DJ (July 1982). "Effect of acyclovir on the deoxyribonucleoside triphosphate pool levels in Vero cells infected with herpes simplex virus type 1". Am. J. Med. 73 (1A): 14–7. doi:10.1016/0002-9343(82)90056-0. PMID 6285704.