DNA origami
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
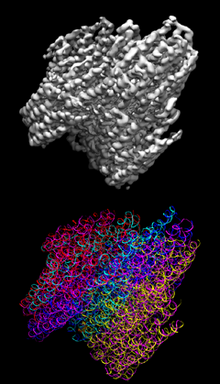
DNA origami is the nanoscale folding of DNA to create arbitrary two- and three-dimensional shapes at the nanoscale. The specificity of the interactions between complementary base pairs make DNA a useful construction material, through design of its base sequences.[2] DNA is a well-understood material that is suitable for creating scaffolds that hold other molecules in place or to create structures all on its own.
DNA origami was the cover story of Nature on March 16, 2006.[3] Since then, DNA origami has progressed past an art form and has found a number of applications from drug delivery systems to uses as circuitry in plasmonic devices; however, most commercial applications remain in a concept or testing phase.[4]
Overview[edit]
The idea of using DNA as a construction material was first introduced in the early 1980s by Nadrian Seeman.[5] The method of DNA origami was developed by Paul Rothemund at the California Institute of Technology.[6] In contrast to common top-down fabrication methods such as 3D printing or lithography which involve depositing or removing material through a tool, DNA Nanotechnology, as well as DNA Origami as a subset, is a bottom-up fabrication method. By rationally designing the constituent subunits of the DNA polymer, DNA can self-assemble into a variety of shapes. The process of constructing DNA Origami involves the folding of a long single strand of viral DNA (typically the 7,249 bp genomic DNA of M13 bacteriophage) aided by multiple smaller "staple" strands. These shorter strands bind the longer in various places, resulting in the formation of a pre-defined two- or three-dimensional shape.[7] Examples include a smiley face and a coarse map of China and the Americas, along with many three-dimensional structures such as cubes.[8]
One of the advantages of using a DNA Origami nanostructure over an otherwise classified DNA nanostructure is the ease of defining finite structures.[9] In the design of some other DNA nanostructures, it can be impractical to design the extremely large number of individualized strands if the entire structure is composed of smaller strands. One method of bypassing the need for a huge number of different strands is to use repeating units, which comes with the disadvantage of a distribution of sizes and sometimes shapes. DNA Origami, however, forms discrete structures.[9]
Applications for DNA Origami are primarily focused around the ability to exert fine control on systems, especially by constraining positions of molecules, typically by attachment to the DNA Origami nanostructures. Current applications are primarily focused around sensing and drug delivery, but many additional applications have been investigated.
To produce a desired shape, images are drawn with a raster fill of a single long DNA molecule. This design is then fed into a computer program that calculates the placement of individual staple strands. Each staple binds to a specific region of the DNA template, and thus due to Watson–Crick base pairing, the necessary sequences of all staple strands are known and displayed. The DNA is mixed, then heated and cooled. As the DNA cools, the various staples pull the long strand into the desired shape. Designs are directly observable via several methods, including electron microscopy, atomic force microscopy, or fluorescence microscopy when DNA is coupled to fluorescent materials.[6]

Bottom-up self-assembly methods are considered promising alternatives that offer cheap, parallel synthesis of nanostructures under relatively mild conditions.
Since the creation of this method, software was developed to assist the process using CAD software. This allows researchers to use a computer to determine the way to create the correct staples needed to form a certain shape. One such software called caDNAno is an open source software for creating such structures from DNA. The use of software has not only increased the ease of the process but has also drastically reduced the errors made by manual calculations.[10][5]
Dynamic Structures and Modifications[edit]
As in the broader field of DNA nanotechnology, DNA Origami may be made dynamic in nature through the use of a variety of methods. The three primary methods of creating a dynamic DNA Origami machine are toehold mediated strand displacement, enzymatic reactions, and base stacking.[11] While these methods are most commonly used, additional methods for creating dynamic DNA Origami machines exist, such as designing a directional component and using brownian motion to drive rotational movement of structures [12] or leveraging less commonly used DNA self-assembly phenomena like G-quadruplexes or i-motifs which can be pH sensitive.[13]

Modifications can be otherwise used to affect structural properties, to impart unique chemistry to the nanostructures, or to add stimuli responses to the nanostructures. Modifications to structures can be made through conjugation of molecules such as proteins, or through chemical modification of the DNA bases themselves. pH dependent responses, light dependent responses, and more have been shown through modified systems.
One example application of creating dynamic structures is the ability to have a stimuli response resulting in drug release, which is presented by several groups.[14][15] Other, less common applications comes in sensing moving mechanisms in vivo such as the unwinding of helicase.[16]
Biomedical Applications[edit]
DNA Origami, being made of a natural biological polymer, is well suited to the biological environment when salt concentrations allow[1], and offers fine control over the positioning of molecules and structures in the system. This allows DNA Origami to be applicable to a number of scenarios in biomedical engineering. Current biomedical applications include drug release with 0 order mechanisms[2], vaccines[3], cell signaling[4], and sensing applications[5].
DNA is folded into an octahedron and coated with a single bilayer of phospholipid, mimicking the envelope of a virus particle. The DNA nanoparticles, each at about the size of a virion, are able to remain in circulation for hours after injected into mice. It also elicits much lower immune response than the uncoated particles. It presents a potential use in drug delivery, reported by researchers in Wyss Institute at Harvard University.[17][18]
Researchers at the Harvard University Wyss Institute reported the self-assembling and self-destructing drug delivery vessels using the DNA origami in the lab tests. The DNA nanorobot they created is an open DNA tube with a hinge on one side which can be clasped shut. The drug filled DNA tube is held shut by a DNA aptamer, configured to identify and seek certain diseased related protein. Once the origami nanobots get to the infected cells, the aptamers break apart and release the drug. The first disease model the researchers used was leukemia and lymphoma.[19]
Researchers in the National Center for Nanoscience and Technology in Beijing and Arizona State University reported a DNA origami delivery vehicle for Doxorubicin, a well-known anti-cancer drug. The drug was non-covalently attached to DNA origami nanostructures through intercalation and a high drug load was achieved. The DNA-Doxorubicin complex was taken up by human breast adenocarcinoma cancer cells (MCF-7) via cellular internalization with much higher efficiency than doxorubicin in free form. The enhancement of cell killing activity was observed not only in regular MCF-7, more importantly, also in doxorubicin-resistant cells. The scientists theorized that the doxorubicin-loaded DNA origami inhibits lysosomal acidification, resulting in cellular redistribution of the drug to action sites, thus increasing the cytotoxicity against the tumor cells.[20][21] Further testing on in vivo on mice suggests that over a 12 day period, Doxorubicin was more effective at reducing tumor sizes in mice when it was contained in DNA Origami Nanostructures or DONs.[22]
Researchers from the Massachusetts Institute of Technology are developing a method to attach various viral antigens to Virus-shaped DNA particles to mimic the virus to be used to develop new vaccines.[23] This was started in 2016 when Bathe’s lab created an algorithm known as DAEDALUS (DNA Origami Sequence Design Algorithm for User-defined Structures) to generate precision-controlled three-dimensional shapes of DNA.[24] Using the tool they designed virus-shaped scaffolding that can modularly attach different antigens to the surface of the DNA scaffold. Currently, MIT is working to develop optimal geometries for B cells to recognize HIV antigens. Further research has attempted to replace HIV antigens with SARS-CoV-2 and are testing whether vaccines show proper immune response from isolated B cells and in mice.[25]

Similarly, researchers from the Technical University of Munich have developed a method to have T-cells target tumor cells by using antigen coated DNA origami.[26] The researchers developed a method to create chassis known as programable T-cell Engagers or (PTEs) which are DNA Origami structures that can be configured to bind to user-defined target cells and T-cells based on which antigens are coated on the surfaces of the nanostructure. The in vitro results show that after 24 hours of exposure 90% of the tumor cells were destroyed. Meanwhile in vivo testing showed that their PTEs were capable of binding to the target proteins for several hours which validates the mechanism they designed.[27]
Nanotechnology Applications[edit]
Many potential applications have been suggested in literature, including enzyme immobilization, drug delivery systems, and nanotechnological self-assembly of materials. Though DNA is not the natural choice for building active structures for nanorobotic applications, due to its lack of structural and catalytic versatility, several papers have examined the possibility of molecular walkers on origami and switches for algorithmic computing.[8][28] The following paragraphs list some of the reported applications conducted in the laboratories with clinical potential.
In a study conducted by a group of scientists from iNANO center and CDNA Center at Aarhus university, researchers were able to construct a small multi-switchable 3D DNA Box Origami. The proposed nanoparticle was characterized by AFM, TEM and FRET. The constructed box was shown to have a unique reclosing mechanism, which enabled it to repeatedly open and close in response to a unique set of DNA or RNA keys. The authors proposed that this "DNA device can potentially be used for a broad range of applications such as controlling the function of single molecules, controlled drug delivery, and molecular computing.".[29]
Nanorobots made of DNA origami demonstrated computing capacities and completed pre-programmed task inside the living organism was reported by a team of bioengineers at Wyss Institute at Harvard University and Institute of Nanotechnology and Advanced Materials at Bar-Ilan University. As a proof of concept, the team injected various kinds of nanobots (the curled DNA encasing molecules with fluorescent markers) into live cockroaches. By tracking the markers inside the cockroaches, the team found the accuracy of delivery of the molecules (released by the uncurled DNA) in target cells, the interactions among the nanobots and the control are equivalent to a computer system. The complexity of the logic operations, the decisions and actions, increases with the increased number of nanobots. The team estimated that the computing power in the cockroach can be scaled up to that of an 8-bit computer.[30][31]
A research group at the Indian Institute of Science used nanostructures to develop a platform to elucidate the coaxial stacking between DNA bases. This approach utilized DNA-PAINT based super-resolution microscopy for visualizing these DNA nanostructures and performed DNA binding kinetics analysis to elucidate the fundamental force of base-stacking that helps stabilize the DNA double helical structure. They went on to assemble multimeric DNA origami nanostructures termed as a 'three-point star' into a tetrahedral 3D origami structure. The assembly relied chiefly on base-stacking interactions between each subunit. The group further showed that the knowledge of such interactions can be used to predict and thus tune the relative stabilities of these multimeric DNA nanostructures.[32]
Similar approaches[edit]
The idea of using protein design to accomplish the same goals as DNA origami has surfaced as well. Researchers at the National Institute of Chemistry in Slovenia are working on using rational design of protein folding to create structures much like those seen with DNA origami. The main focus of current research in protein folding design is in the drug delivery field, using antibodies attached to proteins as a way to create a targeted vehicle.[33][34]
See also[edit]
References[edit]
- ^ a b Bai, Xiao-chen; Martin, Thomas G.; Scheres, Sjors H. W.; Dietz, Hendrik (2012-12-04). "Cryo-EM structure of a 3D DNA-origami object". Proceedings of the National Academy of Sciences. 109 (49): 20012–20017. doi:10.1073/pnas.1215713109. ISSN 0027-8424. PMC 3523823. PMID 23169645.
- ^ a b Zadegan, R.M.; Norton, M.L. (2012). "Structural DNA Nanotechnology: From Design to Applications". Int. J. Mol. Sci. 13 (6): 7149–7162. doi:10.3390/ijms13067149. PMC 3397516. PMID 22837684.
- ^ a b Rothemund, Paul W. K. (2006). "Folding DNA to create nanoscale shapes and patterns". Nature. 440 (7082): 297–302. Bibcode:2006Natur.440..297R. doi:10.1038/nature04586. PMID 16541064. S2CID 4316391.
- ^ a b Sanderson, Katharine (2010). "Bioengineering: What to make with DNA origami". Nature. 464 (7286): 158–159. doi:10.1038/464158a. PMID 20220817.
- ^ a b c Seeman, Nadrian C. (1982-11-21). "Nucleic acid junctions and lattices". Journal of Theoretical Biology. 99 (2): 237–247. Bibcode:1982JThBi..99..237S. doi:10.1016/0022-5193(82)90002-9. PMID 6188926.
- ^ a b Rothemund, Paul W. K. (2006). "Folding DNA to create nanoscale shapes and patterns" (PDF). Nature. 440 (7082): 297–302. Bibcode:2006Natur.440..297R. doi:10.1038/nature04586. ISSN 0028-0836. PMID 16541064. S2CID 4316391.
- ^ Douglas, Shawn M.; Dietz, Hendrik; Liedl, Tim; Högberg, Björn; Graf, Franziska; Shih, William M. (May 2009). "Self-assembly of DNA into nanoscale three-dimensional shapes". Nature. 459 (7245): 414–418. Bibcode:2009Natur.459..414D. doi:10.1038/nature08016. ISSN 0028-0836. PMC 2688462. PMID 19458720.
- ^ a b Lin, Chenxiang; Liu, Yan; Rinker, Sherri; Yan, Hao (2006). "DNA Tile Based Self-Assembly: Building Complex Nanoarchitectures". ChemPhysChem. 7 (8): 1641–7. doi:10.1002/cphc.200600260. PMID 16832805.
- ^ a b Seeman, Nadrian C.; Sleiman, Hanadi F. (November 2017). "DNA nanotechnology". Nature Reviews Materials. 3 (17068). doi:10.1038/natrevmats.2017.68.
- ^ Douglas, Shawn M.; Marblestone, Adam H.; Teerapittayanon, Surat; Vazquez, Alejandro; Church, George M.; Shih, William M. (2009-08-01). "Rapid prototyping of 3D DNA-origami shapes with caDNAno". Nucleic Acids Research. 37 (15): 5001–5006. doi:10.1093/nar/gkp436. ISSN 0305-1048. PMC 2731887. PMID 19531737.
- ^ Hong, Fan; Zhang, Fei; Liu, Yan; Yan, Hao (2017-06-12). "DNA Origami: Scaffolds for Creating Higher Order Structures". ACS Publications. 117 (20): 12584–12640. doi:10.1021/acs.chemrev.6b00825.
- ^ Pumm, Anna-Katharina; Engelen, Wouter; Enzo, Kopperger; Isensee, Jonas; Vogt, Matthias; Kozina, Viktorija; Kube, Massimo; Honemann, Maximilian N.; Bertosin, Eva; Langecker, Martin; Golestanian, Ramin; Simmel, Friedrich C.; Dietz, Hendrik (2022-07-20). "A DNA origami rotary ratchet motor". Nature. 607: 492–498. doi:10.1038/s41586-022-04910-y.
- ^ Julin, Sofia; Linko, Veikko; Kostiainen, Mauri A. (2023-05-31). "Reconfigurable pH-Responsive DNA Origami Lattices". ACS Publications. 17 (11): 11014–11022. doi:10.1021/acsnano.3c03438.
- ^ Bujold, Katherine E.; Hsu, John C. C.; Sleiman, Hanadi F. (2016-10-04). "Optimized DNA "Nanosuitcases" for Encapsulation and Conditional Release of siRNA". ACS Publications. 138 (42): 14030–14038.
- ^ Afonin, Kirill A.; Dobrovolskaia, Marina A.; Church, George; Bathe, Mark (2020-07-24). "Opportunities, Barriers, and a Strategy for Overcoming Translational Challenges to Therapeutic Nucleic Acid Nanotechnology". ACS Publications. 14 (2): 9221–9227.
- ^ Kosuri, Pallav; Altheimer, Benjamin D.; Dai, Mingjie; Zhuang, Xiaowei (2019-07-17). "Rotation tracking of genome-processing enzymes using DNA origami rotors". Nature. 572 (136–140).
- ^ Gibney, Michael (23 April 2014). "DNA nanocages that act like viruses bypass the immune system to deliver drugs". fiercedrugdelivery.com. Archived from the original on 20 September 2015. Retrieved 19 June 2014.
- ^ Perrault, S; Shih, W (2014). "Virus-Inspired Membrane Encapsulation of DNA Nanostructures To Achieve In Vivo Stability". ACS Nano. 8 (5): 5132–5140. doi:10.1021/nn5011914. PMC 4046785. PMID 24694301.
- ^ Garde, Damian (May 15, 2012). "DNA origami could allow for 'autonomous' delivery". fiercedrugdelivery.com. Archived from the original on September 24, 2015. Retrieved May 25, 2012.
- ^ "Folded DNA becomes Trojan horse to attack cancer". New Scientist. 18 August 2012. Retrieved 22 August 2012.
- ^ Jiang, Qiao; Song, Chen; Nangreave, Jeanette; Liu, Xiaowei; Lin, Lin; Qiu, Dengli; Wang, Zhen-Gang; Zou, Guozhang; Liang, Xingjie; Yan, Hao; Ding, Baoquan (2012). "DNA Origami as a Carrier for Circumvention of Drug Resistance". Journal of the American Chemical Society. 134 (32): 13396–13403. doi:10.1021/ja304263n. PMID 22803823.
- ^ Zhang, Qian; Jiang, Qiao; Li, Na; Dai, Luru; Liu, Qing; Song, Linlin; Wang, Jinye; Li, Yaqian; Tian, Jie; Ding, Baoquan; Du, Yang (2014-07-22). "DNA Origami as an In Vivo Drug Delivery Vehicle for Cancer Therapy". ACS Nano. 8 (7): 6633–6643. doi:10.1021/nn502058j. ISSN 1936-0851.
- ^ "Engineers use "DNA origami" to identify vaccine design rules". MIT News | Massachusetts Institute of Technology. 2020-06-29. Retrieved 2024-04-26.
- ^ "Automating DNA origami opens door to many new uses". MIT News | Massachusetts Institute of Technology. 2016-05-26. Retrieved 2024-04-26.
- ^ Veneziano, Rémi; Ratanalert, Sakul; Zhang, Kaiming; Zhang, Fei; Yan, Hao; Chiu, Wah; Bathe, Mark (2016-06-24). "Designer nanoscale DNA assemblies programmed from the top down". Science. 352 (6293): 1534–1534. doi:10.1126/science.aaf4388. ISSN 0036-8075. PMC 5111087. PMID 27229143.
- ^ Munich, Ludwig Maximilian University of. "Artificial DNA structures fitted with antibodies may instruct the immune system to target cancerous cells". phys.org. Retrieved 2024-04-26.
- ^ Wagenbauer, Klaus F.; Pham, Nhi; Gottschlich, Adrian; Kick, Benjamin; Kozina, Viktorija; Frank, Christopher; Trninic, Daniela; Stömmer, Pierre; Grünmeier, Ruth; Carlini, Emanuele; Tsiverioti, Christina Angeliki; Kobold, Sebastian; Funke, Jonas J.; Dietz, Hendrik (November 2023) [2023-08-17]. "Programmable multispecific DNA-origami-based T-cell engagers". Nature Nanotechnology. 18 (11): 1319–1326. doi:10.1038/s41565-023-01471-7. ISSN 1748-3395.
- ^ DNA 'organises itself' on silicon,BBC News, August 17, 2009
- ^ M. Zadegan, Reza; et, al. (2012). "Construction of a 4 Zeptoliters Switchable 3D DNA Box Origami". ACS Nano. 6 (11): 10050–10053. doi:10.1021/nn303767b. PMID 23030709.
- ^ Spickernell, Sarah (8 April 2014). "DNA nanobots deliver drugs in living cockroaches". New Scientist. 222 (2964): 11. Bibcode:2014NewSc.222...11S. doi:10.1016/S0262-4079(14)60709-0. Retrieved 9 June 2014.
- ^ Amir, Y; Ben-Ishay, E; Levner, D; Ittah, S; Abu-Horowitz, A; Bachelet, I (2014). "Universal computing by DNA origami robots in a living animal". Nature Nanotechnology. 9 (5): 353–357. Bibcode:2014NatNa...9..353A. doi:10.1038/nnano.2014.58. PMC 4012984. PMID 24705510.
- ^ Banerjee, Abhinav; Anand, Micky; Kalita, Simanta; Ganji, Mahipal (2023). "Single-molecule analysis of DNA base-stacking energetics using patterned DNA nanostructures". Nature Nanotechnology. 18 (12): 1474–1482. Bibcode:2023NatNa..18.1474B. doi:10.1038/s41565-023-01485-1. ISSN 1748-3387. PMC 10716042. PMID 37591937.
- ^ Peplow, Mark (28 April 2013). "Protein gets in on DNA's origami act". Nature. doi:10.1038/nature.2013.12882. S2CID 87992174.
- ^ Zadegan, Reza M.; Norton, Michael L. (June 2012). "Structural DNA Nanotechnology: From Design to Applications". Int. J. Mol. Sci. 13 (6): 7149–7162. doi:10.3390/ijms13067149. PMC 3397516. PMID 22837684.
Further reading[edit]
- Kube, Massimo; Kohler, Fabian; Feigl, Elija; Nagel-Yüksel, Baki; Willner, Elena M.; Funke, Jonas J.; Gerling, Thomas; Stömmer, Pierre; Honemann, Maximilian N.; Martin, Thomas G.; Scheres, Sjors H. W.; Dietz, Hendrik (December 2020). "Revealing the structures of megadalton-scale DNA complexes with nucleotide resolution". Nature Communications. 11 (1): 6229. Bibcode:2020NatCo..11.6229K. doi:10.1038/s41467-020-20020-7. PMC 7718922. PMID 33277481.