Melibiose
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
| Names | |
|---|---|
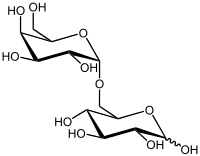
| IUPAC name 6-O-α-D-Galactopyranosyl-β-D-glucopyranose | |
| Systematic IUPAC name (2R,3R,4S,5S,6R)-6-[[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxymethyl]oxane-2,3,4,5-tetrol | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Melibiose |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.297 g·mol−1 |
| Melting point | 84–85 °C (183–185 °F; 357–358 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Melibiose is a reducing disaccharide formed by an α-1,6 linkage between galactose and glucose (D-Gal-(α1→6)-D-Glc).[1][2] It differs from lactose in the chirality of the carbon where the galactose ring is closed and that the galactose is linked to a different point on the glucose moiety. It can be formed by invertase-mediated hydrolysis of raffinose, which produces melibiose and fructose. Melibiose can be broken down into its component saccharides, glucose and galactose, by the enzyme alpha-galactosidase, such as MEL1 from Saccharomyces pastorianus (lager yeast).
Melibiose cannot be used by Saccharomyces cerevisiae[3] (ale yeast), so this is one test to differentiate between the two yeast species.
References
[edit]- ^ Thisbe K. Lindhorst (2007). Essentials of Carbohydrate Chemistry and Biochemistry (1 ed.). Wiley-VCH. ISBN 978-3527315284.
- ^ John F. Robyt (1997). Essentials of Carbohydrate Chemistry (1 ed.). Springer. ISBN 0387949518.
- ^ Bokulicha. Nicholas A. & Bamforth. Charles W. (1 June 2013). "The Microbiology of Malting and Brewing". American Society for Microbiology. pp. 157–172. Retrieved 2 May 2015.