Mercury(II) acetate
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
 | |
| Names | |
|---|---|
| Other names mercuric acetate mercuriacetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.014.993 |
| EC Number |
|
PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1629 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C4H6O4Hg | |
| Molar mass | 318.678 g/mol |
| Appearance | white solid |
| Odor | mild vinegar odor |
| Density | 3.28 g/cm3, solid |
| Melting point | 179 °C (354 °F; 452 K) (decomposes) |
| 25 g/100 mL (10 °C) 100 g/100 mL (100 °C) | |
| Solubility | soluble in alcohol, diethyl ether |
| −100·10−6 cm3/mol | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H300, H310, H330, H373, H410 | |
| P260, P262, P264, P270, P271, P273, P280, P284, P301+P310, P302+P350, P304+P340, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 40.9 mg/kg (rat, oral) 23.9 mg/kg (mouse, oral)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
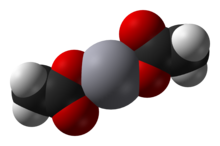
Mercury(II) acetate, also known as mercuric acetate is a chemical compound, the mercury(II) salt of acetic acid, with the formula Hg(O2CCH3)2. Commonly abbreviated Hg(OAc)2, this compound is employed as a reagent to generate organomercury compounds from unsaturated organic precursors. It is a white, water-soluble solid, but some samples can appear yellowish with time owing to decomposition.
Structure
[edit]Mercury(II) acetate is a crystalline solid consisting of isolated Hg(OAc)2 molecules with Hg-O distances of 2.07 Å. Three long, weak intermolecular Hg···O bonds of about 2.75 Å are also present, resulting in a slightly distorted square pyramidal coordination geometry at Hg.[2]
Synthesis and reactions
[edit]Mercury(II) acetate can be produced by reaction of mercuric oxide with acetic acid.[3]
HgO + 2 CH3COOH → Hg(CH3COO)2 + H2O
Inorganic reactions
[edit]Mercury(II) acetate in acetic acid solution reacts with H2S to rapidly precipitate the black (β) polymorph of HgS. With gentle heating of the slurry, the black solid converts to the red form.[4] The mineral cinnabar is red HgS. The precipitation of HgS as well as a few other sulfides, using hydrogen sulfide is a step in qualitative inorganic analysis.
Organic chemistry
[edit]Electron-rich arenes undergo "mercuration" upon treatment with Hg(OAc)2. This behavior is illustrated with phenol:
- C6H5OH + Hg(OAc)2 → C6H4(OH)-2-HgOAc + HOAc
The acetate group (OAc) that remains on mercury can be displaced by chloride:[5]
- C6H4(OH)-2-HgOAc + NaCl → C6H4(OH)-2-HgCl + NaOAc
The Hg2+ center binds to alkenes, inducing the addition of hydroxide and alkoxide. For example, treatment of methyl acrylate with mercuric acetate in methanol gives an α-mercuri ester:[6]
- Hg(OAc)2 + CH2=CHCO2CH3 + CH3OH → CH3OCH2CH(HgOAc)CO2CH3 + HOAc
Exploiting the high affinity of mercury(II) for sulfur ligands, Hg(OAc)2 can be used as a reagent to deprotect thiol groups in organic synthesis. Similarly Hg(OAc)2 has been used to convert thiocarbonate esters into dithiocarbonates:
- (RS)2C=S + H2O + Hg(OAc)2 → (RS)2C=O + HgS + 2 HOAc
Mercury(II) acetate is used for oxymercuration reactions.
A famous use of Hg(OAc)2 was in the synthesis of idoxuridine.
Toxicity
[edit]Mercuric acetate is a highly toxic compound, due to it being water-soluble and having mercury ions. Symptoms of mercury poisoning include peripheral neuropathy, skin discoloration and dequamation (peeling and/or shedding of the skin).[7] Chronic exposure may cause reduced intelligence and kidney failure.[8]
References
[edit]- ^ "Mercury (organo) alkyl compounds (as Hg)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Allmann, R. (1973). "Die Struktur des Quecksilber(II)-acetats". Zeitschrift für Kristallographie - Crystalline Materials. 138 (1–6): 366–373. doi:10.1524/zkri.1973.138.jg.366. S2CID 96160619.
- ^ F. Wagenknecht; R. Juza (1963). "Mercury(II) Acetate". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 2. NY, NY: Academic Press. p. 1120.
- ^ Newell, Lyman C.; Maxson, R. N.; Filson, M. H. (1939). "Red Mercuric Sulfide". Inorganic Syntheses. Vol. 1. pp. 19–20. doi:10.1002/9780470132326.ch7. ISBN 9780470132326.
- ^ Whitmore, F. C.; Hanson, E. R. (1925). "o-Chloromercuriphenol". Organic Syntheses. 4: 13. doi:10.15227/orgsyn.004.0013.
- ^ Carter, Herbert E.; West, Harold D. (1940). "DL-Serine". Organic Syntheses. 20: 81. doi:10.15227/orgsyn.020.0081.
- ^ Bernhoft, Robin A. (2012). "Mercury Toxicity and Treatment: A Review of the Literature". Journal of Environmental and Public Health. 2012: 1–10. doi:10.1155/2012/460508. ISSN 1687-9805. PMC 3253456. PMID 22235210.
- ^ Bose-O'Reilly, Stephan; McCarty, Kathleen M.; Steckling, Nadine; Lettmeier, Beate (September 2010). "Mercury Exposure and Children's Health". Current Problems in Pediatric and Adolescent Health Care. 40 (8): 186–215. doi:10.1016/j.cppeds.2010.07.002. PMC 3096006. PMID 20816346.
