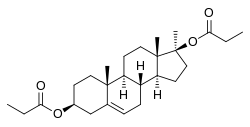
Methandriol dipropionate
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
| Clinical data | |
|---|---|
| Trade names | Arbolic, Durabolic, Or-Bolic, Probolik, Protabolin |
| Other names | Methylandrostenediol dipropionate; Methylandrostenediol 3β,17β-dipropionate; MADP; 17α-Methylandrost-5-ene-3β,17β-diol 3,17β-dipropionate |
| Routes of administration | Intramuscular injection[1] |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.669 |
| Chemical and physical data | |
| Formula | C26H40O4 |
| Molar mass | 416.602 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Methandriol dipropionate (MADP), also known as methylandrostenediol dipropionate and sold under the brand names Arbolic, Durabolic, Or-Bolic, Probolik, and Protabolin among others, is a synthetic, injected anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of 5-androstenediol.[2][1] It is an androgen ester – specifically, the C3,17β dipropionate ester of methandriol (17α-methyl-5-androstenediol) – and acts as a prodrug of methandriol in the body.[2][1] Methandriol dipropionate is administered by intramuscular injection and, relative to methandriol, has an extended duration via this route of several days due to a depot effect afforded by its ester.[1] It was marketed in the United States,[2] but is no longer available in this country.[3]
See also
[edit]- List of androgen esters § Esters of other synthetic AAS
- Estradiol benzoate/progesterone/methandriol dipropionate
References
[edit]- ^ a b c d Llewellyn W (1 November 2008). Anabolics: Anabolic Steroid Reference Guide. William Llewellyn. pp. 294–296. ISBN 978-0-9679304-7-3.
- ^ a b c Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 663–. ISBN 978-3-88763-075-1.
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 29 November 2016.
External links
[edit]- "Methandriol (methandriol, methandriol dipropionate)". William Llewellyn's Anabolic.org. Archived from the original on 2019-06-29.