NFEPP

 | |
| Clinical data | |
|---|---|
| Drug class | Opioid |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
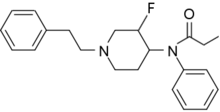
| Formula | C22H27FN2O |
| Molar mass | 354.469 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
NFEPP (N-(3-fluoro-1-phenethylpiperidin-4-yl)-N-phenylpropionamide) is an analgesic opioid chemical, similar in structure to fentanyl, designed in 2016 by Spahn et al. from Free University of Berlin[2] to avoid the standard negative side effects of opiates, including opioid overdose, by only targeting inflamed tissue.[3][4]
Inflamed tissue
[edit]Inflamed tissue has a lower pH value (~5–7) than non-inflamed tissue (7.4).[5] Through computer simulation, scientists found a way to make the fentanyl analog only affect inflamed tissue via the addition of fluorine to the chemical structure. In experiment, it was shown that NFEPP produced injury-restricted analgesia in rats with different types of inflammatory pain without exhibiting typical opiate effects, including respiratory depression, sedation, constipation, and chemical seeking behavior.[6][7][8]
As a result, NFEPP has the potential to reduce opioid addiction and dependency, as there is no effect on users who are not actually suffering from pain, as the chemical does not interact with non-inflamed brain tissue until much higher doses are reached.[9]
References
[edit]- ^ Drug Enforcement Administration Do (February 2018). "Schedules of Controlled Substances:Temporary Placement of Fentanyl-Related Substances in Schedule I. Temporary amendment; temporary scheduling order". Federal Register. 83 (25): 5188–92. PMID 29932611.
- ^ Spahn V, Del Vecchio G, Labuz D, Rodriguez-Gaztelumendi A, Massaly N, Temp J, et al. (March 2017). "A nontoxic pain killer designed by modeling of pathological receptor conformations". Science. 355 (6328): 966–969. Bibcode:2017Sci...355..966S. doi:10.1126/science.aai8636. PMID 28254944. S2CID 206653322.
- ^ Halford B (2017). "An opioid minus major side effects". Chemical & Engineering News. 95 (10): 8.
- ^ Edwards SR, Blough BE, Cowart K, Howell GH, Araujo AA, Haskell JP, et al. (May 2024). "Assessment of the Antinociceptive, Respiratory-Depressant, and Reinforcing Effects of the Low pKa Fluorinated Fentanyl Analogs, FF3 and NFEPP". Neuropharmacology: 110002. doi:10.1016/j.neuropharm.2024.110002. PMID 38754577.
- ^ Mole B (4 March 2017). "Early study suggests new opioid is non-addictive, works only where it hurt". Ars Technica.
- ^ Rodriguez-Gaztelumendi A, Spahn V, Labuz D, Machelska H, Stein C (November 2018). "Analgesic effects of a novel pH-dependent μ-opioid receptor agonist in models of neuropathic and abdominal pain". Pain. 159 (11): 2277–2284. doi:10.1097/j.pain.0000000000001328. PMC 6203420. PMID 29994988.
- ^ Massaly N, Temp J, Machelska H, Stein C (December 2020). "Uncovering the analgesic effects of a pH-dependent mu-opioid receptor agonist using a model of nonevoked ongoing pain". Pain. 161 (12): 2798–2804. doi:10.1097/j.pain.0000000000001968. PMID 32639370. S2CID 220410251.
- ^ Degro CE, Jiménez-Vargas NN, Tsang Q, Yu Y, Guzman-Rodriguez M, Alizadeh E, et al. (June 2023). "Evolving acidic microenvironments during colitis provide selective analgesic targets for a pH-sensitive opioid". Pain. 164 (11): 2501–2515. doi:10.1097/j.pain.0000000000002956. PMC 10731875. PMID 37326658.
{{cite journal}}: CS1 maint: PMC embargo expired (link) - ^ Baamonde A, Menéndez L, González-Rodríguez S, Lastra A, Seitz V, Stein C, et al. (October 2020). "A low pKa ligand inhibits cancer-associated pain in mice by activating peripheral mu-opioid receptors". Scientific Reports. 10 (1): 18599. Bibcode:2020NatSR..1018599B. doi:10.1038/s41598-020-75509-4. PMC 7596718. PMID 33122720.