Nylon

This article's lead section may be too short to adequately summarize the key points. (September 2023) |
 Nylon 6,6 Nylon 6,6  | |
|---|---|
| Density | 1.15 g/cm3 |
| Electrical conductivity (σ) | 10−12 S/m |
| Thermal conductivity | 0.25 W/(m·K) |
| Melting point | 463–624 K 190–350 °C 374–663 °F |
Nylon is a family of synthetic polymers with amide backbones, usually linking aliphatic or semi-aromatic groups.
Nylons are white or colorless[1][2] and soft; some are silk-like.[3] They are thermoplastic, which means that they can be melt-processed into fibers, films, and diverse shapes.[4][5][6]: 2 The properties of nylons are often modified by blending with a wide variety of additives.
Many kinds of nylon are known. One family, designated nylon-XY, is derived from diamines and dicarboxylic acids of carbon chain lengths X and Y, respectively. An important example is nylon-6,6 (C₁₂H₂₂N₂O₂). Another family, designated nylon-Z, is derived from aminocarboxylic acids with carbon chain length Z. An example is nylon-[6].
Nylon polymers have significant commercial applications in fabric and fibers (apparel, flooring and rubber reinforcement), in shapes (molded parts for cars, electrical equipment, etc.), and in films (mostly for food packaging).[7]
History
[edit]
DuPont and the invention of nylon
[edit]Researchers at DuPont began developing cellulose-based fibers, culminating in the synthetic fiber rayon. DuPont's experience with rayon was an important precursor to its development and marketing of nylon.[8]: 8, 64, 236
DuPont's invention of nylon spanned an eleven-year period, ranging from the initial research program in polymers in 1927 to its announcement in 1938, shortly before the opening of the 1939 New York World's Fair.[9] The project grew from a new organizational structure at DuPont, suggested by Charles Stine in 1927, in which the chemical department would be composed of several small research teams that would focus on "pioneering research" in chemistry and would "lead to practical applications".[8]: 92 Harvard instructor Wallace Hume Carothers was hired to direct the polymer research group. Initially he was allowed to focus on pure research, building on and testing the theories of German chemist Hermann Staudinger.[10] He was very successful, as research he undertook greatly improved the knowledge of polymers and contributed to the science.[11]
Nylon was the first commercially successful synthetic thermoplastic polymer.[12] DuPont began its research project in 1927.[9] The first nylon, nylon 66, was synthesized on February 28, 1935, by Wallace Hume Carothers at DuPont's research facility at the DuPont Experimental Station.[13][14] In response to Carothers' work, Paul Schlack at IG Farben developed nylon 6, a different molecule based on caprolactam, on January 29, 1938.[15]: 10 [16]
In the spring of 1930, Carothers and his team had already synthesized two new polymers. One was neoprene, a synthetic rubber greatly used during World War II.[17] The other was a white elastic but strong paste that would later become nylon. After these discoveries, Carothers' team was made to shift its research from a more pure research approach investigating general polymerization to a more practically focused goal of finding "one chemical combination that would lend itself to industrial applications".[8]: 94
It was not until the beginning of 1935 that a polymer called "polymer 6-6" was finally produced. Carothers' coworker, Washington University alumnus Julian W. Hill had used a cold drawing method to produce a polyester in 1930.[18] This cold drawing method was later used by Carothers in 1935 to fully develop nylon.[19] The first example of nylon (nylon 6.6) was produced on February 28, 1935, at DuPont's research facility at the DuPont Experimental Station.[13] It had all the desired properties of elasticity and strength. However, it also required a complex manufacturing process that would become the basis of industrial production in the future. DuPont obtained a patent for the polymer in September 1938,[20] and quickly achieved a monopoly of the fiber.[11] Carothers died 16 months before the announcement of nylon, therefore he was never able to see his success.[9]
Nylon was first used commercially in a nylon-bristled toothbrush in 1938,[5][21] followed more famously in women's stockings or "nylons" which were shown at the 1939 New York World's Fair and first sold commercially in 1940,[22] whereupon they became an instant commercial success with 64 million pairs sold during their first year on the market. During World War II, almost all nylon production was diverted to the military for use in parachutes and parachute cord. Wartime uses of nylon and other plastics greatly increased the market for the new materials.[23]
The production of nylon required interdepartmental collaboration between three departments at DuPont: the Department of Chemical Research, the Ammonia Department, and the Department of Rayon.[24] Some of the key ingredients of nylon had to be produced using high pressure chemistry, the main area of expertise of the Ammonia Department. Nylon was considered a "godsend to the Ammonia Department",[8] which had been in financial difficulties. The reactants of nylon soon constituted half of the Ammonia Department's sales and helped them come out of the period of the Great Depression by creating jobs and revenue at DuPont.[8]
DuPont's nylon project demonstrated the importance of chemical engineering in industry, helped create jobs, and furthered the advancement of chemical engineering techniques. In fact, it developed a chemical plant that provided 1800 jobs and used the latest technologies of the time, which are still used as a model for chemical plants today.[8] The ability to acquire a large number of chemists and engineers quickly was a huge contribution to the success of DuPont's nylon project.[8]: 100–101 The first nylon plant was located at Seaford, Delaware, beginning commercial production on December 15, 1939. On October 26, 1995, the Seaford plant was designated a National Historic Chemical Landmark by the American Chemical Society.[25]
Early marketing strategies
[edit]An important part of nylon's popularity stems from DuPont's marketing strategy. DuPont promoted the fiber to increase demand before the product was available to the general market. Nylon's commercial announcement occurred on October 27, 1938, at the final session of the Herald Tribune's yearly "Forum on Current Problems", on the site of the approaching New York City world's fair.[10][11]: 141 The "first man-made organic textile fiber" which was derived from "coal, water and air" and promised to be "as strong as steel, as fine as the spider's web" was received enthusiastically by the audience, many of them middle-class women, and made the headlines of most newspapers.[11]: 141 Nylon was introduced as part of "The world of tomorrow" at the 1939 New York World's Fair[26] and was featured at DuPont's "Wonder World of Chemistry" at the Golden Gate International Exposition in San Francisco in 1939.[10][27] Actual nylon stockings were not shipped to selected stores in the national market until May 15, 1940. However, a limited number were released for sale in Delaware before that.[11]: 145–146 The first public sale of nylon stockings occurred on October 24, 1939, in Wilmington, Delaware. 4,000 pairs of stockings were available, all of which were sold within three hours.[10]
Another added bonus to the campaign was that it meant reducing silk imports from Japan, an argument that won over many wary customers. Nylon was even mentioned by President Roosevelt's cabinet, which addressed its "vast and interesting economic possibilities" five days after the material was formally announced.[11]
However, the early excitement over nylon also caused problems. It fueled unreasonable expectations that nylon would be better than silk, a miracle fabric as strong as steel that would last forever and never run.[11]: 145–147 [22] Realizing the danger of claims such as "New Hosiery Held Strong as Steel" and "No More Runs", DuPont scaled back the terms of the original announcement, especially those stating that nylon would possess the strength of steel.[11]
Also, DuPont executives marketing nylon as a revolutionary man-made material did not at first realize that some consumers experienced a sense of unease and distrust, even fear, towards synthetic fabrics.[11]: 126–128 A particularly damaging news story, drawing on DuPont's 1938 patent for the new polymer, suggested that one method of producing nylon might be to use cadaverine (pentamethylenediamine),[a] a chemical extracted from corpses. Although scientists asserted that cadaverine was also extracted by heating coal, the public often refused to listen. A woman confronted one of the lead scientists at DuPont and refused to accept that the rumour was not true.[11]: 146–147
DuPont changed its campaign strategy, emphasizing that nylon was made from "coal, air and water", and started focusing on the personal and aesthetic aspects of nylon, rather than its intrinsic qualities.[11]: 146–147 Nylon was thus domesticated,[11]: 151–152 and attention shifted to the material and consumer aspect of the fiber with slogans like "If it's nylon, it's prettier, and oh! How fast it dries!".[8]: 2
Production of nylon fabric
[edit]
After nylon's nationwide release in 1940, its production ramped up significantly. In that year alone, 1300 tons of the fabric were produced, marking a remarkable start for this innovative material.[8]: 100 The demand for nylon surged, particularly for nylon stockings, which became an instant sensation. During their first year on the market, an astounding 64 million pairs of nylon stockings were sold, reflecting the fabric's rapid integration into daily life and fashion.[8]: 101 Such was the success of nylon that in 1941, just a year after its launch, a second plant was opened in Martinsville, Virginia, to meet the growing demand and ensure a steady supply of this popular fabric. This expansion underscored the profound impact nylon had on the textile industry and its rapid rise to prominence as a versatile and sought-after material.[28]


While nylon was marketed as the durable and indestructible material of the people, it was sold at about one-and-a-half times the price of silk stockings ($4.27 per pound of nylon versus $2.79 per pound of silk).[8]: 101 Sales of nylon stockings were strong in part due to changes in women's fashion. As Lauren Olds explains: "by 1939 [hemlines] had inched back up to the knee, closing the decade just as it started off". The shorter skirts were accompanied by a demand for stockings that offered fuller coverage without the use of garters to hold them up.[29]
However, as of February 11, 1942, nylon production was redirected from being a consumer material to one used by the military.[10] DuPont's production of nylon stockings and other lingerie stopped, and most manufactured nylon was used to make parachutes and tents for World War II.[30] Although nylon stockings already made before the war could be purchased, they were generally sold on the black market for as high as $20.[28]
Once the war ended, the return of nylon was awaited with great anticipation. Although DuPont projected yearly production of 360 million pairs of stockings, there were delays in converting back to consumer rather than wartime production.[10] In 1946, the demand for nylon stockings could not be satisfied, which led to the nylon riots. In one instance, an estimated 40,000 people lined up in Pittsburgh to buy 13,000 pairs of nylons.[22] In the meantime, women cut up nylon tents and parachutes left from the war in order to make blouses and wedding dresses.[31][32] Between the end of the war and 1952, production of stockings and lingerie used 80% of the world's nylon. DuPont put focus on catering to the civilian demand, and continually expanded its production.
Introduction of nylon blends
[edit]As pure nylon hosiery was sold in a wider market, problems became apparent. Nylon stockings were found to be fragile, in the sense that the thread often tended to unravel lengthwise, creating 'runs'.[8]: 101 People also reported that pure nylon textiles could be uncomfortable due to nylon's lack of absorbency.[33] Moisture stayed inside the fabric near the skin under hot or moist conditions instead of being "wicked" away.[34] Nylon fabric could also be itchy and tended to cling and sometimes spark as a result of static electrical charge built up by friction.[35][36] Also, under some conditions, stockings could decompose[11] turning back into nylon's original components of air, coal, and water. Scientists explained this as a result of air pollution, attributing it to London smog in 1952, as well as poor air quality in New York and Los Angeles.[37][38][39]
The solution found to problems with pure nylon fabric was to blend nylon with other existing fibers or polymers such as cotton, polyester, and spandex. This led to the development of a wide array of blended fabrics. The new nylon blends retained the desirable properties of nylon (elasticity, durability, ability to be dyed) and kept clothes prices low and affordable.[30]: 2 As of 1950, the New York Quartermaster Procurement Agency (NYQMPA), which developed and tested textiles for the Army and Navy, had committed to developing a wool-nylon blend. They were not the only ones to introduce blends of both natural and synthetic fibers. America's Textile Reporter referred to 1951 as the "Year of the blending of the fibers".[40] Fabric blends included mixes like "Bunara" (wool-rabbit-nylon) and "Casmet" (wool-nylon-fur).[41] In Britain, in November 1951, the inaugural address of the 198th session of the Royal Society for the Encouragement of Arts, Manufactures and Commerce focused on the blending of textiles.[42]
DuPont's Fabric Development Department cleverly targeted French fashion designers, supplying them with fabric samples. In 1955, designers such as Coco Chanel, Jean Patou, and Christian Dior showed gowns created with DuPont fibers, and fashion photographer Horst P. Horst was hired to document their use of DuPont fabrics.[22] American Fabrics credited blends with providing "creative possibilities and new ideas for fashions which had been hitherto undreamed of."[41]
Etymology
[edit]DuPont went through an extensive process to generate names for its new product.[11]: 138–139 In 1940, John W. Eckelberry of DuPont stated that the letters "nyl" were arbitrary, and the "on" was copied from the suffixes of other fibers such as cotton and rayon. A later publication by DuPont (Context, vol. 7, no. 2, 1978) explained that the name was originally intended to be "No-Run" ("run" meaning "unravel") but was modified to avoid making such an unjustified claim. Since the products were not really run-proof, the vowels were swapped to produce "nuron", which was changed to "nilon" "to make it sound less like a nerve tonic". For clarity in pronunciation, the "i" was changed to "y".[22][43]
A persistent urban legend exists that the name is derived from "New York" and "London"; however, no organisation in London was ever involved in the research and production of nylon.[44]
Longer-term popularity
[edit]Nylon’s popularity soared in the 1940s and 1950s due to its durability and sheerness. In the 1970s, it became more popular due to its flexibility and price.
In spite of oil shortages in the 1970s, consumption of nylon textiles continued to grow by 7.5% per year between the 1960s and 1980s.[45] Overall production of synthetic fibers, however, dropped from 63% of the worlds textile production in 1965, to 45% of the world's textile production in early 1970s.[45] The appeal of "new" technologies wore off, and nylon fabric "was going out of style in the 1970s".[8] Also, consumers became concerned about environmental costs throughout the production cycle: obtaining the raw materials (oil), energy use during production, waste produced during creation of the fiber, and eventual waste disposal of materials that were not biodegradable.[45] Synthetic fibers have not dominated the market since the 1950s and 1960s. As of 2020[update], the worldwide production of nylon is estimated at 8.9 million tons.[46]
Although pure nylon has many flaws and is now rarely used, its derivatives have greatly influenced and contributed to society. From scientific discoveries relating to the production of plastics and polymerization, to economic impact during the depression and the changing of women's fashion, nylon was a revolutionary product.[22] The Lunar Flag Assembly, the first flag planted on the moon in a symbolic gesture of celebration, was made of nylon. The flag itself cost $5.50 but had to have a specially designed flagpole with a horizontal bar so that it would appear to "fly".[47][48] One historian describes nylon as "an object of desire", comparing the invention to Coca-Cola in the eyes of 20th century consumers.[8]
Chemistry
[edit]| External videos | |
|---|---|
In common usage, the prefix "PA" (polyamide) or the name "Nylon" are used interchangeably and are equivalent in meaning.
The nomenclature used for nylon polymers was devised during the synthesis of the first simple aliphatic nylons and uses numbers to describe the number of carbons in each monomer unit, including the carbon(s) of the carboxylic acid(s).[49][50] Subsequent use of cyclic and aromatic monomers required the use of letters or sets of letters. One number after "PA" or "Nylon" indicates a homopolymer which is monadic or based on one amino acid (minus H2O) as monomer:
- PA 6 or Nylon 6: [NH−(CH2)5−CO]n made from ε-caprolactam.
Two numbers or sets of letters indicate a dyadic homopolymer formed from two monomers: one diamine and one dicarboxylic acid. The first number indicates the number of carbons in the diamine. The two numbers should be separated by a comma for clarity, but the comma is often omitted.
- PA or Nylon 6,10 (or 610): [NH−(CH2)6−NH−CO−(CH2)8−CO]n made from hexamethylenediamine and sebacic acid;
For copolymers the comonomers or pairs of comonomers are separated by slashes:
- PA 6/66: [NH−(CH2)6−NH−CO−(CH2)4−CO]n−[NH−(CH2)5−CO]m made from caprolactam, hexamethylenediamine and adipic acid;
- PA 66/610: [NH−(CH2)6−NH−CO−(CH2)4−CO]n−[NH−(CH2)6−NH−CO−(CH2)8−CO]m made from hexamethylenediamine, adipic acid and sebacic acid.
The term polyphthalamide (abbreviated to PPA) is used when 60% or more moles of the carboxylic acid portion of the repeating unit in the polymer chain is composed of a combination of terephthalic acid (TPA) and isophthalic acid (IPA).
Types
[edit]Nylon 66 and related heteropolymers
[edit]Nylon 66 and related polyamides are condensation polymers forms from equal parts of diamine and dicarboxylic acids.[51] In the first case, the "repeating unit" has the ABAB structure, as also seen in many polyesters and polyurethanes. Since each monomer in this copolymer has the same reactive group on both ends, the direction of the amide bond reverses between each monomer, unlike natural polyamide proteins, which have overall directionality: C terminal → N terminal. In the second case (so called AA), the repeating unit corresponds to the single monomer.[15]: 45–50 [52]
Wallace Carothers at DuPont patented nylon 66.[20][53][54] In the case of nylons that involve reaction of a diamine and a dicarboxylic acid, it is difficult to get the proportions exactly correct, and deviations can lead to chain termination at molecular weights less than a desirable 10,000 daltons. To overcome this problem, a crystalline, solid "nylon salt" can be formed at room temperature, using an exact 1:1 ratio of the acid and the base to neutralize each other. The salt is crystallized to purify it and obtain the desired precise stoichiometry. Heated to 285 °C (545 °F), the salt reacts to form nylon polymer with the production of water.
Nylon 510, made from pentamethylene diamine and sebacic acid, was included in the Carothers patent to nylon 66[20] Nylon 610 is produced similarly using hexamethylene diamine. These materials are more expensive because of the relatively high cost of sebacic acid. Owing to the high hydrocarbon content, nylon 610 is more hydrophobic and finds applications suited for this property, such as bristles.[55]
| 1,4-diaminobutane | 1,5-diaminopentane | MPMD | HMD | MXD | Nonanediamine | Decanediamine | Dodecanediamine | Bis(para-aminocyclohexyl)methane | Trimethylhexamethylenediamine | |
|---|---|---|---|---|---|---|---|---|---|---|
| Adipic acid | 46 | D6 | 66 | MXD6 | ||||||
| Sebacic acid | 410 | 510 | 610 | 1010 | ||||||
| Dodecanedioic acid | 612 | 1212 | PACM12 | |||||||
| Terephthalic acid | 4T | DT | 6T | 9T | 10T | 12T | TMDT | |||
| Isophthalic acid | DI | 6I |
Examples of these polymers that are or were commercially available:
Nylon 6 and related homopolymers
[edit]These polymers are made from a lactam or amino acid. The synthetic route using lactams (cyclic amides) was developed by Paul Schlack at IG Farben, leading to nylon 6, or polycaprolactam—formed by a ring-opening polymerization. The peptide bond within the caprolactam is broken with the exposed active groups on each side being incorporated into two new bonds as the monomer becomes part of the polymer backbone.
The 428 °F (220 °C) melting point of nylon 6 is lower than the 509 °F (265 °C) melting point of nylon 66.[60] Homopolymer nylons are derived from one monomer.
| Monomer | Polymer |
|---|---|
| Caprolactam | 6 |
| 11-aminoundecanoic acid | 11 |
| ω-aminolauric acid | 12 |
Examples of these polymers that are or were commercially available:
Nylon 1,6
[edit]Nylons can also be synthesized from dinitriles using acid catalysis. For example, this method is applicable for preparation of nylon 1,6 from adiponitrile, formaldehyde and water.[64] Additionally, nylons can be synthesized from diols and dinitriles using this method as well.[65]
Copolymers
[edit]It is easy to make mixtures of the monomers or sets of monomers used to make nylons to obtain copolymers. This lowers crystallinity and can therefore lower the melting point.
Some copolymers that have been or are commercially available are listed below:
- PA6/66 DuPont Zytel[66]
- PA6/6T BASF Ultramid T (6/6T copolymer)[67]
- PA6I/6T DuPont Selar PA[68]
- PA66/6T DuPont Zytel HTN[67]
- PA12/MACMI EMS Grilamid TR[69]
Blends
[edit]Most nylon polymers are miscible with each other allowing a range of blends to be made. The two polymers can react with one another by transamidation to form random copolymers.[70]
Crystallinity
[edit]According to their crystallinity, polyamides can be:
- semi-crystalline:
- high crystallinity: PA46 and PA66;
- low crystallinity: PAMXD6 made from m-xylylenediamine and adipic acid;
- amorphous: PA6I made from hexamethylenediamine and isophthalic acid.
According to this classification, PA66, for example, is an aliphatic semi-crystalline homopolyamide.
Environmental impact
[edit]
All nylons are susceptible to hydrolysis, especially by strong acids, a reaction essentially the reverse of their synthesis. The molecular weight of nylon products so attacked drops, and cracks form quickly at the affected zones. Lower members of the nylons (such as nylon 6) are affected more than higher members such as nylon 12. This means that nylon parts cannot be used in contact with sulfuric acid for example, such as the electrolyte used in lead–acid batteries.
When being molded, nylon must be dried to prevent hydrolysis in the molding machine barrel since water at high temperatures can also degrade the polymer.[71] The reaction is shown above.
The average greenhouse gas footprint of nylon in manufacturing carpets is estimated at 5.43 kg CO2 equivalent per kg, when produced in Europe. This gives it almost the same carbon footprint as wool, but with greater durability and therefore a lower overall carbon footprint.[72]
Data published by PlasticsEurope indicates for nylon 66 a greenhouse gas footprint of 6.4 kg CO2 equivalent per kg, and an energy consumption of 138 kJ/kg.[73] When considering the environmental impact of nylon, it is important to consider the use phase.
Various nylons break down in fire and form hazardous smoke, and toxic fumes or ash, typically containing hydrogen cyanide. Incinerating nylons to recover the high energy used to create them is usually expensive, so most nylons reach the garbage dumps, decaying slowly.[b] Discarded nylon fabric takes 30–40 years to decompose.[74] Nylon used in discarded fishing gear such as fishing nets is a contributor to debris in the ocean.[75] Nylon is a robust polymer and lends itself well to recycling. Much nylon resin is recycled directly in a closed loop at the injection molding machine, by grinding sprues and runners and mixing them with the virgin granules being consumed by the molding machine.[76]
Because of the expense and difficulties of the nylon recycling process, few companies utilize it while most favor using cheaper, newly made plastics for their products instead.[75] US clothing company Patagonia has products containing recycled nylon and in the mid-2010s invested in Bureo, a company that recycles nylon from used fishing nets to use in sunglasses and skateboards.[75] The Italian company Aquafil also has demonstrated recycling fishing nets lost in the ocean into apparel.[77] Vanden Recycling recycles nylon and other polyamides (PA) and has operations in the UK, Australia, Hong Kong, the UAE, Turkey and Finland.[78]
Nylon is the most popular fiber type in the residential carpet industry today.[79] The US EPA estimates that 9.2% of carpet fiber, backing and padding was recycled in 2018, 17.8% was incinerated in waste-to-energy facilities, and 73% was discarded in landfills.[80] Some of the world's largest carpet and rug companies are promoting "cradle to cradle"—the re-use of non-virgin materials including ones not historically recycled—as the industry's pathway forward.[81][82]
Properties
[edit]Above their melting temperatures, Tm, thermoplastics like nylon are amorphous solids or viscous fluids in which the chains approximate random coils. Below Tm, amorphous regions alternate with regions which are lamellar crystals.[83] The amorphous regions contribute elasticity, and the crystalline regions contribute strength and rigidity. The planar amide (-CO-NH-) groups are very polar, so nylon forms multiple hydrogen bonds among adjacent strands. Because the nylon backbone is so regular and symmetrical, especially if all the amide bonds are in the trans configuration, nylons often have high crystallinity and make excellent fibers. The amount of crystallinity depends on the details of formation, as well as on the kind of nylon.
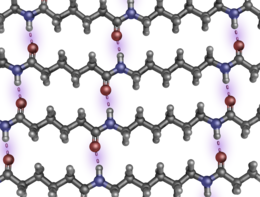
Nylon 66 can have multiple parallel strands aligned with their neighboring peptide bonds at coordinated separations of exactly six and four carbons for considerable lengths, so the carbonyl oxygens and amide hydrogens can line up to form interchain hydrogen bonds repeatedly, without interruption (see the figure opposite). Nylon 510 can have coordinated runs of five and eight carbons. Thus parallel (but not antiparallel) strands can participate in extended, unbroken, multi-chain β-pleated sheets, a strong and tough supermolecular structure similar to that found in natural silk fibroin and the β-keratins in feathers. (Proteins have only an amino acid α-carbon separating sequential -CO-NH- groups.) Nylon 6 will form uninterrupted H-bonded sheets with mixed directionalities, but the β-sheet wrinkling is somewhat different. The three-dimensional disposition of each alkane hydrocarbon chain depends on rotations about the 109.47° tetrahedral bonds of singly bonded carbon atoms.
When extruded into fibers through pores in an industry spinneret, the individual polymer chains tend to align because of viscous flow. If subjected to cold drawing afterwards, the fibers align further, increasing their crystallinity, and the material acquires additional tensile strength. In practice, nylon fibers are most often drawn using heated rolls at high speeds.[84]
Block nylon tends to be less crystalline, except near the surfaces due to shearing stresses during formation. Nylon is clear and colorless, or milky, but is easily dyed. Multistranded nylon cord and rope is slippery and tends to unravel. The ends can be melted and fused with a heat source such as a flame or electrode to prevent this.
Nylons are hygroscopic and will absorb or desorb moisture as a function of the ambient humidity. Variations in moisture content have several effects on the polymer. Firstly, the dimensions will change, but more importantly moisture acts as a plasticizer, lowering the glass transition temperature (Tg), and consequently the elastic modulus at temperatures below the Tg[85]
When dry, polyamide is a good electrical insulator. However, polyamide is hygroscopic. The absorption of water will change some of the material's properties such as its electrical resistance. Nylon is less absorbent than wool or cotton.
The characteristic features of nylon 66 include:
- Pleats and creases can be heat-set at higher temperatures
- More compact molecular structure
- Better weathering properties; better sunlight resistance
- Softer "Hand"
- High melting point (256 °C, 492.8 °F)
- Superior colorfastness
- Excellent abrasion resistance
On the other hand, nylon 6 is easy to dye, more readily fades; it has a higher impact resistance, a more rapid moisture absorption, greater elasticity, and elastic recovery.
- Variation of luster: nylon has the ability to be very lustrous, semi-lustrous, or dull.
- Durability: its high tenacity fibers are used for seatbelts, tire cords, ballistic cloth, and other uses.
- High elongation
- Excellent abrasion resistance
- Highly resilient (nylon fabrics are heat-set)
- Paved the way for easy-care garments
- High resistance to insects, fungi, animals, as well as molds, mildew, rot, and many chemicals
- Used in carpets and nylon stockings
- Melts instead of burning
- Used in many military applications
- Good specific strength
- Transparent to infrared light (−12 dB)[86][clarification needed]
Nylon clothing tends to be less flammable than cotton and rayon, but nylon fibers may melt and stick to skin.[87][88]
Uses
[edit]Nylon was first used commercially in a nylon-bristled toothbrush in 1938,[5][21] followed more famously in women's stockings or "nylons" which were shown at the 1939 New York World's Fair and first sold commercially in 1940.[22] Its use increased dramatically during World War II, when the need for fabrics increased dramatically.
Fibers
[edit]

Bill Pittendreigh, DuPont, and other individuals and corporations worked diligently during the first few months of World War II to find a way to replace Asian silk and hemp with nylon in parachutes. It was also used to make tires, tents, ropes, ponchos, and other military supplies. It was even used in the production of a high-grade paper for U.S. currency. At the outset of the war, cotton accounted for more than 80% of all fibers used and manufactured, and wool fibers accounted for nearly all of the rest. By August 1945, manufactured fibers had taken a market share of 25%, at the expense of cotton. After the war, because of shortages of both silk and nylon, nylon parachute material was sometimes repurposed to make dresses.[89]
Nylon 6 and 66 fibers are used in carpet manufacture.
Nylon is one kind of fiber used in tire cord. Herman E. Schroeder pioneered application of nylon in tires.
Molds and resins
[edit]Nylon resins are widely used in the automobile industry especially in the engine compartment.[90][6]: 514
Molded nylon is used in hair combs and mechanical parts such as machine screws, gears, gaskets, and other low- to medium-stress components previously cast in metal.[91][92] Engineering-grade nylon is processed by extrusion, casting, and injection molding. Type 6,6 Nylon 101 is the most common commercial grade of nylon, and Nylon 6 is the most common commercial grade of molded nylon.[93][94] For use in tools such as spudgers, nylon is available in glass-filled variants which increase structural and impact strength and rigidity, and molybdenum disulfide-filled variants which increase lubricity. Nylon can be used as the matrix material in composite materials, with reinforcing fibers like glass or carbon fiber; such a composite has a higher density than pure nylon.[95] Such thermoplastic composites (25% to 30% glass fiber) are frequently used in car components next to the engine, such as intake manifolds, where the good heat resistance of such materials makes them feasible competitors to metals.[96]
Nylon was used to make the stock of the Remington Nylon 66 rifle.[97] The frame of the modern Glock pistol is made of a nylon composite.[98]
Food packaging
[edit]Nylon resins are used as a component of food packaging films where an oxygen barrier is needed.[7] Some of the terpolymers based upon nylon are used every day in packaging. Nylon has been used for meat wrappings and sausage sheaths.[99] The high temperature resistance of nylon makes it useful for oven bags.[100]
Filaments
[edit]Nylon filaments are primarily used in brushes especially toothbrushes[5] and string trimmers. They are also used as monofilaments in fishing line. Nylon 610 and 612 are the most used polymers for filaments.
Its various properties also make it very useful as a material in additive manufacturing; specifically, as a filament in consumer and professional grade fused deposition modeling 3D printers.
Other forms
[edit]Nylon resins can be extruded into rods, tubes, and sheets.[6]: 209
Nylon powders are used to powder coat metals. Nylon 11 and nylon 12 are the most widely used.[6]: 53
In the mid-1940s, classical guitarist Andrés Segovia mentioned the shortage of good guitar strings in the United States, particularly his favorite Pirastro catgut strings, to a number of foreign diplomats at a party, including General Lindeman of the British Embassy. A month later, the General presented Segovia with some nylon strings which he had obtained via some members of the DuPont family. Segovia found that although the strings produced a clear sound, they had a faint metallic timbre which he hoped could be eliminated.[101] Nylon strings were first tried on stage by Olga Coelho in New York in January 1944.[102] In 1946, Segovia and string maker Albert Augustine were introduced by their mutual friend Vladimir Bobri, editor of Guitar Review. On the basis of Segovia's interest and Augustine's past experiments, they decided to pursue the development of nylon strings. DuPont, skeptical of the idea, agreed to supply the nylon if Augustine would endeavor to develop and produce the actual strings. After three years of development, Augustine demonstrated a nylon first string whose quality impressed guitarists, including Segovia, in addition to DuPont.[101] Wound strings, however, were more problematic. Eventually, however, after experimenting with various types of metal and smoothing and polishing techniques, Augustine was also able to produce high quality nylon wound strings.[101]
See also
[edit]- Ballistic nylon – Thick, tough, nylon fabric
- Cordura – Brand of high-performance fabrics developed by DuPont and now owned by Invista
- Forensic engineering – Investigation of failures associated with legal intervention
- Nylon-eating bacteria – Species of bacteria
- Polyamide – Macromolecule with repeating units linked by amide bonds
- Ripstop nylon – Reinforced woven fabric
- Step-growth polymerization – Type of polymerization reaction mechanism
Notes
[edit]- ^ Actually the most common nylon polymers are made from hexamethylenediamine, with one more CH2 group than cadaverine.
- ^ Typically 80 to 100% is sent to landfill or garbage dumps, while less than 18% are incinerated while recovering the energy. See Francesco La Mantia (August 2002). Handbook of plastics recycling. iSmithers Rapra Publishing. pp. 19–. ISBN 978-1-85957-325-9.
References
[edit]- ^ Clark, Jim. "Polyamides". Chemguide. Retrieved 27 January 2015.
- ^ "Nylon". Encyclopedia Britannica. Retrieved 2020-12-30.
- ^ Lew, Darrin (2021-04-19). "Theoretical Comparison Between Nylon and Silk - Global Warming". Dr. Darrin Lew. Retrieved 2021-06-24.
- ^ Vogler, H. (2013). "Wettstreit um die Polyamidfasern". Chemie in unserer Zeit. 47 (1): 62–63. doi:10.1002/ciuz.201390006.
- ^ a b c d "Nylon, a Petroleum Polymer". American Oil and Gas Historical Society. Retrieved 21 June 2017.
- ^ a b c d Kohan, Melvin (1995). Nylon Plastics Handbook. Munich: Carl Hanser Verlag. ISBN 1569901899.
- ^ a b "Nylons (Polyamide)". British Plastics Federation. Retrieved 19 June 2017.
- ^ a b c d e f g h i j k l Ndiaye, Pap A.; Forster, Elborg (2007). Nylon and bombs : DuPont and the march of modern America. Baltimore: Johns Hopkins University Press. p. 182. ISBN 9780801884443. Retrieved 19 June 2017.
- ^ a b c DuPont (1988). Nylon: A DuPont Invention. DuPont International, Public Affairs. pp. 2–3.
- ^ a b c d e f Kativa, Hillary (2016). "Synthetic Threads". Distillations. 2 (3): 16–21. Retrieved 20 March 2018.
- ^ a b c d e f g h i j k l m n Meikle, Jeffrey L. (1995). American plastic: A cultural history (1. ppb. print ed.). New Brunswick, NJ: Rutgers University Press. ISBN 0813522358.
- ^ "Science of Plastics". Science History Institute. 2016-07-18. Retrieved 26 March 2018.
- ^ a b American Chemical Society National Historic Chemical Landmarks. "Foundations of Polymer Science: Wallace Hume Carothers and the Development of Nylon". ACS Chemistry for Life. Retrieved 27 January 2015.
- ^ "Wallace Hume Carothers". Science History Institute. June 2016. Retrieved 20 March 2018.
- ^ a b McIntyre, J. E. (2005). Synthetic fibres: nylon, polyester, acrylic, polyolefin (1st ed.). Cambridge: Woodhead. p. 10. ISBN 9780849325922. Retrieved 5 July 2017.
- ^ Travis, Anthony S. (1998). Determinants in the evolution of the European chemical industry: 1900-1939: new technologies, political frameworks, markets and companies. Dordrecht: Kluwer Acad. Publ. p. 115. ISBN 9780792348900. Retrieved 5 July 2017.
- ^ "Neoprene: The First Synthetic Rubber". chlorine.americanchemistry.com. Archived from the original on 2020-09-26. Retrieved 2018-12-06.
- ^ "Wallace Carothers and the Development of Nylon - Landmark". American Chemical Society. Retrieved 2019-08-14.
- ^ Stout, David (1996-02-01). "Julian W. Hill, Nylon's Discoverer, Dies at 91". The New York Times. ISSN 0362-4331. Retrieved 2019-08-14.
- ^ a b c US patent 2130523, Carothers W.H., "Linear polyamides and their production", issued 1938-09-20, assigned to EI Du Pont de Nemours and Co.
- ^ a b Nicholson, Joseph L.; Leighton, George R. (August 1942). "Plastics Come of Age". Harper's Magazine. pp. 300–307. Retrieved 5 July 2017.
- ^ a b c d e f g Wolfe, Audra J. (October 3, 2008). "Nylon: A Revolution in Textiles". Distillations Magazine. Science History Institute. Archived from the original on March 21, 2018. Retrieved 20 March 2018.
- ^ "The History and Future of Plastics". Conflicts in Chemistry: The Case of Plastics. Science History Institute. Archived from the original on 20 March 2018. Retrieved 20 March 2018.
- ^ Nylon and Bombs: DuPont and the March of Modern America. 2007. ISBN 9781421403342. Archived from the original on 2022-08-08. Retrieved 2022-08-08.
{{cite book}}:|website=ignored (help) - ^ McAllister, John F. (Oct 26, 1995). "A National Historic Chemical Landmark: The First Nylon Plant" (PDF). American Chemical Society. Retrieved 26 June 2017.
- ^ Blakinger, Keri (April 30, 2016). "A look back at some of the coolest attractions at the 1939 World's Fair". New York Daily News. Archived from the original on Sep 12, 2017. Retrieved 20 June 2017.
- ^ Sundberg, Richard J. (2017). The Chemical Century: Molecular Manipulation and Its Impact on the 20th Century. Apple Academic Press, Incorporated. ISBN 9781771883665.
- ^ a b Colbert, Judy (2013). It Happened in Delaware. Rowman & Littlefield. p. 60. ISBN 978-0-7627-9577-2.
- ^ Olds, Lauren (2001). "World War II and Fashion: The Birth of the New Look". Constructing the Past. 2 (1): Article 6. Retrieved 19 June 2017.
- ^ a b Krier, Beth Ann (27 October 1988). "How Nylon Changed the World : 50 Years Ago Today, It Reshaped the Way We Live--and Think". Los Angeles Times.
- ^ "Parachute Wedding Dress, 1947". Smithsonian National Museum of American History. Retrieved 20 June 2017.
- ^ Woman's Home Companion. 75. Crowell-Collier Publishing Company: 155. 1948.
{{cite journal}}: Missing or empty|title=(help) - ^ Reader's Digest (2002). New complete guide to sewing: step-by-step techniques for making clothes and home accessories. London: Reader's Digest. p. 19. ISBN 9780762104208. Retrieved 26 June 2017.
- ^ "How to buy a trail bed". Backpacker. 5 (3): 70. June 1977. Retrieved 26 June 2017.
- ^ Mendelson, Cheryl (2005). Home comforts : the art and science of keeping house. New York: Scribner. p. 224. ISBN 978-0743272865. Retrieved 26 June 2017.
- ^ Shaeffer, Claire (2008). Claire Shaeffer's fabric sewing guide (2nd ed.). Cincinnati, Ohio: Krause Publications. pp. 88–90. ISBN 978-0896895362.
- ^ Cheremisinoff, Nicholas P. (2002). Handbook of air pollution prevention and control. Amsterdam: Butterworth-Heinemann. p. 65. ISBN 9780080507927.
- ^ Stern, Arthur C., ed. (1970). Air pollution and its effects (2nd ed.). New York: Academic press. p. 72. ISBN 978-0-12-666551-2. Retrieved 26 June 2017.
- ^ Garte, Seymour (2008). Where we stand : a surprising look at the real state of our planet. New York: AMACOM. p. 60. ISBN 978-0814409107. Retrieved 26 June 2017.
- ^ Haggard, John V. (16 May 1957). "Chapter III: Collaborative Procurement of Textiles". Procurement of Clothing and Textiles, 1945-53. 2 (3): 79–84.
- ^ a b Handley, Susannah (1999). Nylon: The Story of a Fashion Revolution. Baltimore, MD: Johns Hopkins University Press. p. 68. ISBN 978-0756771720. Retrieved 26 June 2017.
- ^ Goodale, Ernest W. (16 November 1951). "The Blending & Mixture of Textile Fibres & Yarns". Journal of the Royal Society of Arts. 100 (4860): 4–15. JSTOR 41368063.
- ^ Algeo, John (2009). The Origins and Development of the English Language. Vol. 6. Cengage. p. 224. ISBN 9781428231450.
- ^ Wilton, David (2008). Word Myths: Debunking Linguistic Urban Legends. Oxford University Press. p. 88. ISBN 978-0-199-74083-3.
- ^ a b c Wilson, Sheena; Carlson, Adam; Szeman, Imre (2017). Petrocultures: Oil, Politics, Culture. Montreal, Quebec: McGill-Queen's University Press. p. 246. ISBN 9780773550391. Retrieved 26 June 2017.
- ^ "Global Nylon Market Analysis and Outlook 2020-2027 - Nylon 6".
- ^ Welsh, Jennifer (21 May 2016). "The American Flags on the Moon Have All Turned White". Business Insider. Retrieved 14 April 2017.
- ^ Platoff, Anne M. (1993). "NASA Contractor Report 188251 Where No Flag Has Gone Before: Political and Technical Aspects of Placing a Flag on the Moon". NASA. Retrieved 26 June 2017.
- ^ Cowie, J. M. G. (1991). Polymers: Chemistry and Physics of Modern Materials (2nd ed.). Blackie. pp. 16–17. ISBN 0-216-92980-6.
- ^ Rudin, Alfred (1982). Elements of Polymer Science and Engineering. Academic Press. pp. 32–33. ISBN 0-12-601680-1.
- ^ Ratner, Buddy D. (2013). Biomaterials science : an introduction to materials in medicine (3rd ed.). Amsterdam: Elsevier. pp. 74–77. ISBN 9780080877808. Retrieved 5 July 2017.
- ^ Denby, Derek; Otter, Chris; Stephenson, Kay (2008). Chemical storylines (3rd ed.). Oxford: Heinemann. p. 96. ISBN 9780435631475. Retrieved 5 July 2017.
- ^ "Diamine-dicarboxylic acid salts and process of preparing same US 2130947 A". Patents. Retrieved 19 June 2017.
- ^ "Synthetic fiber US 2130948 A". Patents. Retrieved 19 June 2017.
- ^ Estes, Leland L.; Schweizer, Michael (2011). "Fibers, 4. Polyamide Fibers". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a10_567.pub2. ISBN 978-3527306732.
- ^ "Stanyl® Polyamide 46: Driving change in automotive". DSM. Retrieved 19 June 2017.
- ^ "EcoPaXX: The green performer". DSM. Retrieved 19 June 2017.
- ^ "ForTii® Pushing peak performance". DSM. Retrieved 19 June 2017.
- ^ "zytel - PA6, PA610, PA612, PA66 - dupont". Material Data Center. Retrieved 19 June 2017.
- ^ "Fiber-reinforced composite articles and methods of making them CA 2853925 A1". Patents. Retrieved 19 June 2017.
- ^ "Durethan® is the trade name for our range of engineering thermoplastics based on polyamide 6 and polyamide 66". LANXESS Energizing Chemistry. Retrieved 19 June 2017.
- ^ "Polyamide Resins for an Extreme World Flagship Rilsan® PA11 and Complementary Resins & Alloys". Arkema. Retrieved 19 June 2017.
- ^ "VESTAMID® L—polyamide 12". EVONIK. Retrieved 19 June 2017.
- ^ Magat, Eugene E.; Faris, Burt F.; Reith, John E.; Salisbury, L. Frank (1951-03-01). "Acid-catalyzed Reactions of Nitriles. I. The Reaction of Nitriles with Formaldehyde1". Journal of the American Chemical Society. 73 (3): 1028–1031. doi:10.1021/ja01147a042. ISSN 0002-7863.
- ^ Lakouraj, Moslem Mansour; Mokhtary, Masoud (2009-02-20). "Synthesis of polyamides from p-Xylylene glycol and dinitriles". Journal of Polymer Research. 16 (6): 681. doi:10.1007/s10965-009-9273-z. ISSN 1022-9760. S2CID 98232570.
- ^ "Zytel® 74G33EHSL NC010". DISTRUPOL. Retrieved 19 June 2017.
- ^ a b Kutz, Myer (2011). Applied plastics engineering handbook processing and materials (1st ed.). Amsterdam: William Andrew. p. 5. ISBN 9781437735154. Retrieved 19 June 2017.
- ^ "DuPont TM Selar® PA 2072" (PDF). DuPont. Archived from the original (PDF) on 2015-04-19. Retrieved 19 June 2017.
- ^ "Grilamid L PA12". EMS. Retrieved 19 June 2017.
- ^ Samperi, Filippo; Montaudo, Maurizio S.; Puglisi, Concetto; Di Giorgi, Sabrina; Montaudo, Giorgio (August 2004). "Structural Characterization of Copolyamides Synthesized via the Facile Blending of Polyamides". Macromolecules. 37 (17): 6449–6459. Bibcode:2004MaMol..37.6449S. doi:10.1021/ma049575x.
- ^ "Adhesive for nylon & kevlar". Reltek. Retrieved 27 January 2015.
- ^ Berners-Lee, Mike (2010). How bad are bananas? : the carbon footprint of everything. London: Profile Books. p. 112, table 6.1.
- ^ Eco-profiles and Environmental Product Declarations of the European Plastics Manufacturers: Polyamide 6.6. Brussels: PlasticsEurope AISBL. 2014. Archived from the original on 2015-04-27. Retrieved 2015-04-19.
- ^ "Approximate Time it Takes for Garbage to Decompose in the Environment" (PDF). NH Department of Environmental Services. Archived from the original (PDF) on 2009-04-13. Retrieved 31 March 2018.
- ^ a b c Chhabra, Esha (18 May 2016). "Recycling nylon is good for the planet – so why don't more companies do it?". The Guardian. Retrieved 21 April 2021.
- ^ Boydell, P; Bradfield, C; von Falkenhausen, V; Prautzsch, G (1995). "Recycling of Waste from Glass-reinforced nylon resins". Engineering Design. 2: 8–10.
- ^ Maile, Kelly (January 18, 2019). "How abandoned fishing nets are recycled into nylon". Recycling Today. Retrieved 15 March 2019.
- ^ "PA / Nylon fibres are used in textiles, fishing line and carpets". Vanden Recycling. Retrieved 7 Feb 2020.
- ^ EPA (19 October 2018). "Nylon Carpet: Pros and Cons". Retrieved 27 May 2021.
- ^ EPA (7 September 2017). "Durable Goods: Product-Specific Data (Carpets and Rugs)". Retrieved 27 May 2021.
- ^ Floor covering weekly. "Shaw recognized for Cradle to Cradle commitment". Retrieved 27 May 2021.
- ^ "Cradle To Cradle®". Shaw Industries. Retrieved 27 May 2021.
- ^ Valerie Menzer's Nylon 66 Webpage. Arizona University
- ^ Campbell, Ian M. (2000). Introduction to synthetic polymers. Oxford: Oxford Univ. Press. ISBN 978-0198564706.
- ^ "Measurement of Moisture Effects on the Mechanical Properties of 66 Nylon - TA Instruments Thermal Analysis Application Brief TA-133" (PDF). TA Instruments. Retrieved 19 June 2017.
- ^ Bjarnason, J. E.; Chan, T. L. J.; Lee, A. W. M.; Celis, M. A.; Brown, E. R. (2004). "Millimeter-wave, terahertz, and mid-infrared transmission through common clothing". Applied Physics Letters. 85 (4): 519. Bibcode:2004ApPhL..85..519B. doi:10.1063/1.1771814.
- ^ "Flammable clothing". The Children's Hospital at Westmead. 24 February 2016. Retrieved 5 July 2017.
- ^ Workshop on Mass Burns (1968 : Washington, D.C.) (1969). Phillips, Anne W.; Walter, Carl W. (eds.). Mass burns : proceeding of a workshop, 13-14 March 1968 / sponsored by the Committee on Fire Research, Division of Engineering, National Research Council and the Office of Civil Defense, Dept. of the Army. Washington, D.C.: National Academy of Sciences ; Springfield, Va. : reproduced by the Clearinghouse for Federal Scientific & Technical Information. p. 30. Retrieved 5 July 2017.
{{cite book}}: CS1 maint: numeric names: authors list (link) - ^ Caruso, David (2009). "Saving the (Wedding) Day: Oral History Spotlight" (PDF). Transmutations. Fall (5): 2. Archived from the original (PDF) on May 9, 2016.
- ^ "Engine Oil Pan". www.materialdatacenter.com. Retrieved 19 June 2017.
- ^ "Nylon Machining & Fabrication | ESPE". www.espemfg.com. Retrieved 2018-08-28.
- ^ Youssef, Helmi A.; El-Hofy, Hassan A.; Ahmed, Mahmoud H. (2011). Manufacturing technology : materials, processes, and equipment. Boca Raton, FL: Taylor & Francis/CRC Press. p. 350. ISBN 9781439810859.
- ^ "NYLON 6,6 (Nylon 6)" (PDF). Serrata. Retrieved 19 June 2017.
- ^ "Nylon 6 vs. Nylon 66: What's the Difference?". PolyOne. Retrieved 5 July 2017.
- ^ "Fiberglass and Composite Material Design Guide". Performance Composites Inc. Retrieved 27 January 2015.
- ^ Page, I. B. (2000). Polyamides as engineering thermoplastic materials. Shawbury, Shrewsbury: Rapra Technology Ltd. p. 115. ISBN 9781859572207.
- ^ "How do you take care of a nylon 66 or 77? You don't". Field & Stream. 75 (9). 1971.
- ^ Sweeney, Patrick (2013). Glock deconstructed. Iola, Wis.: Krause. p. 92. ISBN 978-1440232787.
- ^ Colbert, Judy (2013). It happened in Delaware : remarkable events that shaped history (First ed.). Morris Book Publishing. ISBN 978-0-7627-6968-1.
- ^ "Oven Bags". Cooks Info. Retrieved 19 April 2015.
- ^ a b c "The History of Classical guitar strings". Maestros of the Guitar. Retrieved 27 January 2015.
- ^ Bellow, Alexander (1970). The Illustrated History of the Guitar. New York: Franco Colombo. p. 193.
Further reading
[edit]- Kadolph, Sara J. (2007). Textiles. Pearson Prentice Hall. ISBN 978-0-13-118769-6.
- Kohan, Melvin (1995). Nylon Plastics Handbook. Munich: Carl Hanser Verlag. ISBN 1569901899.
- "How Nylon Yarn is Made". Popular Science. December 1946. pp. 132–3.
External links
[edit]- Making Nylon, Bob Burk, CHEM 1000, Carleton University, Ottawa, Canada on YouTube
- Polyamide Nylon Plastic
- Joseph X. Labovsky Collection of Nylon Photographs and Ephemera Science History Institute Digital Collections. (High-resolution scans of nylon-related photographs and ephemera collected by Joseph X. Labovsky, a lab assistant to Wallace Carothers, during the early stages of nylon development and production at DuPont).

