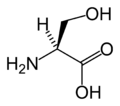
絲氨酸

此條目可参照英語維基百科相應條目来扩充。 |
| 丝氨酸 | |||
|---|---|---|---|
| |||
 | |||
| IUPAC名 Serine | |||
| 别名 | 2-Amino-3-hydroxypropanoic acid | ||
| 识别 | |||
| CAS号 | 56-45-1 ? 302-84-1 312-84-5((D-isomer)) | ||
| PubChem | 617 | ||
| ChemSpider | 5736 (L-form), 597 | ||
| SMILES |
| ||
| InChI |
| ||
| EC编号 | 206-130-6 | ||
| ChEBI | 17115 | ||
| DrugBank | DB00133 | ||
| IUPHAR配体 | 726 | ||
| 性质[2] | |||
| 化学式 | C3H7NO3 | ||
| 摩尔质量 | 105.09 g·mol−1 | ||
| 外观 | 白色晶体或粉末 | ||
| 密度 | 1.603 g/cm3 (22 °C) | ||
| 熔点 | 246 °C 分解 | ||
| 溶解性(水) | 可溶 | ||
| pKa | 2.21 (carboxyl), 9.15 (amino)[1] | ||
| 若非注明,所有数据均出自标准状态(25 ℃,100 kPa)下。 | |||
絲氨酸(英語:serine,三字代码:Ser,一字代码:S)是一種非必需的基本氨基酸,中心碳原子帶有羥甲基側鏈,為極性氨基酸。其密码子为UCU、UCC、UCA、UCG、AGU与AGC。絲氨酸富含於鸡蛋、鱼和黃豆。絲氨酸可促進脂肪和脂肪酸的新陳代謝,有助於維持免疫系統。其在醫藥上有著廣泛用途。
发现
[编辑]
丝氨酸是基本氨基酸之一,自然状态下仅以左旋形式存在于蛋白质中。丝氨酸为非必需氨基酸,其在人体内可通过甘氨酸合成。丝氨酸最先于1865年由德国化学家埃米尔·克莱默从富含该成分的絲蛋白中提取[3],得名自拉丁文中的丝绸,sericum。其化学结构于1902年被测定。[4][5]
合成与化学反应
[编辑]工业上以甘氨酸和甲醇为原料,由羟甲基转移酶催化合成L-丝氨酸[6]。
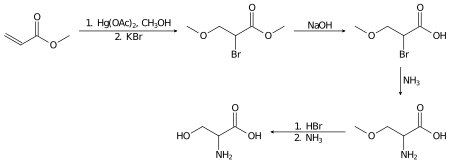
消旋丝氨酸则可以在实验室中,以丙烯酸甲酯为原料,通过以下几步制备[7]:
丝氨酸的加氢反应则生成一种二醇,丝氨醇:
- HOCH
2CH(NH
2)CO
2H + 2 H
2 → HOCH
2CH(NH
2)CH
2OH + 2 H
2O
生物功能
[编辑]新陈代谢
[编辑]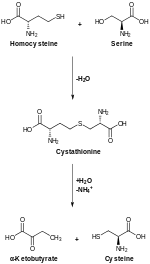
丝氨酸参与多种物质的生物合成,因而在新陈代谢中起重要作用。丝氨酸参与嘌呤与嘧啶的合成,亦是合成多种氨基酸的前体,包括甘氨酸与半胱氨酸,以及细菌合成途径下产生的酪氨酸。丝氨酸亦是鞘脂与叶酸的前体,这两种物质是体内生物合成重要的一碳片段供体。[來源請求]
信号分子
[编辑]D-丝氨酸,作为一种神经调质,可在神经元中由L -丝氨酸经丝氨酸消旋酶催化合成,主要作为NMDA受体上甘氨酸位点(NR1)的强力兴奋剂,使受体在结合谷氨酸激活后得以开放离子通道。对于NMDA受体离子通道的开放,谷氨酸以及甘氨酸(或D-丝氨酸)的结合是必不可少的,除此之外不能有通道阻断剂作用(如Mg2+或Zn2+ )[8]。实际上,D-丝氨酸对于甘氨酸位点的作用比甘氨酸自身更强。[9][10]D-丝氨酸曾被认为只存在于细菌中,直到最近的研究表明其为第二种自然存在于人体内的D 氨基酸(另一种为不久前发现的D- 天冬氨酸),NMDA受体上的甘氨酸位点也可能因此更名D- 丝氨酸位点[11] 。除中枢神经系统外,D -丝氨酸亦可在外周组织发挥信号分子作用,如在软骨[12] 、肾脏[13] 以及阴茎海绵体[14]。
参考文献
[编辑]- ^ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ^ Weast, Robert C. (编). CRC Handbook of Chemistry and Physics 62nd. Boca Raton, FL: CRC Press. 1981: C-512. ISBN 0-8493-0462-8..
- ^ Cramer, Emil. Ueber die Bestandtheile der Seide [On the constituents of silk]. Journal für praktische Chemie. 1865, 96: 76–98 [2023-09-16]. (原始内容存档于2022-11-06) (German). Serine is named on p. 93: "Ich werde den in Frage stehenden Körper unter dem Namen Serin beschreiben." (I will describe the body [i.e., substance] in question by the name "serine".)
- ^ Fischer, Emil; Leuchs, Hermann. Synthese des Serins, der l-Glucosaminsäure und anderer Oxyaminosäuren [Synthesis of serine, of l-glucosaminic acid, and other oxyamino acids]. Berichte der Deutschen Chemischen Gesellschaft. 1902, 35 (3): 3787–3805. doi:10.1002/cber.190203503213 (德语).
- ^ Serine. The Columbia Encyclopedia 6th ed.. encyclopedia.com. [22 October 2012]. (原始内容存档于2016-08-10).
- ^ Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker, Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, 2005, doi:10.1002/14356007.a02_057.pub2
- ^ Carter HE, West HD. dl-Serine. Org. Synth. 1940, 20: 81 [2023-09-19]. doi:10.15227/orgsyn.020.0081. (原始内容存档于2023-01-30).
- ^ Liu Y, Hill RH, Arhem P, von Euler G. NMDA and glycine regulate the affinity of the Mg2+-block site in NR1-1a/NR2A NMDA receptor channels expressed in Xenopus oocytes. Life Sciences. 2001, 68 (16): 1817–1826. PMID 11292060. doi:10.1016/S0024-3205(01)00975-4.
- ^ MacKay, Mary-Anne B.; Kravtsenyuk, Maryana; Thomas, Rejish; Mitchell, Nicholas D.; Dursun, Serdar M.; Baker, Glen B. D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression?. Frontiers in Psychiatry. 6 February 2019, 10: 25. ISSN 1664-0640. PMC 6372501
 . PMID 30787885. doi:10.3389/fpsyt.2019.00025
. PMID 30787885. doi:10.3389/fpsyt.2019.00025  .
. D-Serine is more potent than glycine as a coagonist at the NMDA receptor, has a regional distribution in the brain that is similar to that of NMDA receptors and appears to be more closely associated with synaptic NMDA receptors than glycine (which is more closely associated with non-synaptic NMDA receptors).
- ^ Wolosker, Herman; Balu, Darrick T. D-Serine as the gatekeeper of NMDA receptor activity: implications for the pharmacologic management of anxiety disorders. Translational Psychiatry. 9 June 2020, 10 (1): 184. ISSN 2158-3188. PMC 7283225
 . PMID 32518273. doi:10.1038/s41398-020-00870-x.
. PMID 32518273. doi:10.1038/s41398-020-00870-x. D-Serine is functionally a more potent activator of synaptic NMDARs than glycine, and mounting evidence suggests that it serves as the major NMDAR co-agonist in limbic brain regions implicated in neuropsychiatric disorders.
- ^ Mothet JP, Parent AT, Wolosker H, Brady RO, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-Serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proceedings of the National Academy of Sciences of the United States of America. Apr 2000, 97 (9): 4926–4931. Bibcode:2000PNAS...97.4926M. PMC 18334
 . PMID 10781100. doi:10.1073/pnas.97.9.4926
. PMID 10781100. doi:10.1073/pnas.97.9.4926  .
. - ^ Takarada T, Hinoi E, Takahata Y, Yoneda Y. Serine racemase suppresses chondrogenic differentiation in cartilage in a Sox9-dependent manner. Journal of Cellular Physiology. May 2008, 215 (2): 320–328. PMID 17929246. S2CID 45669104. doi:10.1002/jcp.21310.
- ^ Ma MC, Huang HS, Chen YS, Lee SH. Mechanosensitive N-methyl-D-aspartate receptors contribute to sensory activation in the rat renal pelvis. Hypertension. Nov 2008, 52 (5): 938–944. PMID 18809793. doi:10.1161/HYPERTENSIONAHA.108.114116
 .
. - ^ Ghasemi M, Rezania F, Lewin J, Moore KP, Mani AR. D-Serine modulates neurogenic relaxation in rat corpus cavernosum. Biochemical Pharmacology. Jun 2010, 79 (12): 1791–1796. PMID 20170643. doi:10.1016/j.bcp.2010.02.007.