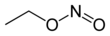
Ethyl nitrite

| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Ethyl nitrite | |||
| Other names 1-Nitrosooxyethane Ethyl alcohol nitrite Nitrous acid Nitrous ether Ethyl ester Nitrethyl | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.385 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C2H5NO2 | |||
| Molar mass | 75.067 g·mol−1 | ||
| Boiling point | 17 °C (63 °F; 290 K) | ||
| 5.07 g/100 ml | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | [1] | ||
| NFPA 704 (fire diamond) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
The chemical compound ethyl nitrite is an alkyl nitrite with a chemical formula C2H5NO2. It may be prepared from ethanol.[2]
Uses
[edit]It is used as a reagent with butanone to yield the dimethylglyoxime end product.
Ethyl nitrite is the main ingredient in a traditional ethanol-based South African remedy for colds and flu known as Witdulsies, which is sold in pharmacies. It is known as a traditional Afrikaans remedy; the same remedy is apparently made by the Amish in the US. However, FDA has blocked over-the-counter sales of this same remedy, known in the US as sweet nitrite or sweet spirit of nitre, since 1980.[3] Its use has been associated with fatal methemoglobinemia.[4]
Methemoglobinemia is the primary toxic effect of ethyl nitrite.[5] Due to ethyl nitrite's high volatility and faint smell, in the presence of ethyl nitrite vapors, it is easy to breath a high dose of it without realizing, resulting in methemoglobinemia,[6] which may or may not be severe, or even fatal.
References
[edit]- ^ "NFPA 704 Ratings for Common Chemicals".
- ^ Semon, W. L.; Damerell, V. R. (1943). "Dimethylglyoxime". Organic Syntheses; Collected Volumes, vol. 2, p. 204.
- ^ "Rulemaking History for OTC Sweet Spirits of Nitre Drug Products". fda.gov. Retrieved 2016-12-26.
- ^ "ETHYL NITRITE - National Library of Medicine HSDB Database". toxnet.nlm.nih.gov. Retrieved 2017-11-18. "Archived copy". Archived from the original on 2017-12-01. Retrieved 2017-11-18.
{{cite web}}: CS1 maint: archived copy as title (link) CS1 maint: bot: original URL status unknown (link) - ^ "Ethyl nitrite". Haz-Map. Retrieved 2020-08-08.
- ^ Titov, V Yu; Petrenko, Yu M (2005). "Proposed mechanism of nitrite-induced methemoglobinemia". Biochemistry (Moscow). 70 (4): 473–83. doi:10.1007/s10541-005-0139-7. PMID 15892615. S2CID 22906218.