HSPA8

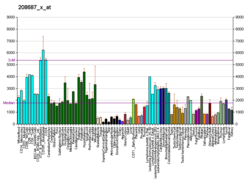
Heat shock 70 kDa protein 8 also known as heat shock cognate 71 kDa protein or Hsc70 or Hsp73 is a heat shock protein that in humans is encoded by the HSPA8 gene on chromosome 11.[5] As a member of the heat shock protein 70 family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins.[5][6] Its functions contribute to biological processes including signal transduction, apoptosis, autophagy, protein homeostasis, and cell growth and differentiation.[6][7][8] It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence, and aging.[6][7]
Structure
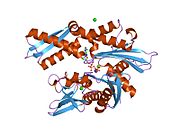
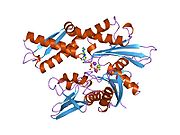
[edit]This gene encodes a 70kDa heat shock protein which is a member of the heat shock protein 70 (Hsp70) family.[5] As a Hsp70 protein, it has a C-terminal protein substrate-binding domain and an N-terminal ATP-binding domain.[9][10][11] The substrate-binding domain consists of two subdomains, a two-layered β-sandwich subdomain (SBDβ) and an α-helical subdomain (SBDα), which are connected by the loop Lα,β. SBDβ contains the peptide binding pocket while SBDα serves as a lid to cover the substrate binding cleft. The ATP binding domain consists of four subdomains split into two lobes by a central ATP/ADP binding pocket. The two terminal domains are linked together by a conserved region referred to as loop LL,1, which is critical for allosteric regulation. The unstructured region at the very end of the C-terminal is believed to be the docking site for co-chaperones.[11]
Function
[edit]The heat shock protein 70 (Hsp70) family contains both heat-inducible and constitutively expressed members. The latter are called heat-shock cognate (Hsc) proteins. The heat shock 70 kDa protein 8 also known as Hsc70 belongs to the heat-shock cognate subgroup. This protein binds to nascent polypeptides to facilitate correct protein folding.[5] In order to properly fold non-native proteins, Hsp70 chaperones interact with the hydrophobic peptide segments of proteins in an ATP-controlled fashion. Though the exact mechanism still remains unclear, there are at least two alternative modes of action: kinetic partitioning and local unfolding. In kinetic partitioning, Hsp70s repetitively bind and release substrates in cycles that maintain low concentrations of free substrate. This effectively prevents aggregation while allowing free molecules to fold to the native state. In local unfolding, the binding and release cycles induce localized unfolding in the substrate, which helps to overcome kinetic barriers for folding to the native state. Ultimately, its role in protein folding contributes to its function in signal transduction, apoptosis, protein homeostasis, and cell growth and differentiation.[6][7] Hsc70 is known to localize to the cytoplasm and lysosome, where it participates in chaperone-mediated autophagy by aiding the unfolding and translocation of substrate proteins across the membrane into the lysosomal lumen.[12][13] Through this pathway, Hsc70 also contributes to the degradation of the proapoptotic BBC3/PUMA under normal conditions, thus conferring cytoprotection.[13]
Hsc70 additionally serves as a positive regulator of cell cycle transition and carcinogenesis. For example, Hsc70 regulates the nuclear accumulation of cyclin D1, which is a key player in G1 to S phase cell cycle transition.[14][15]
Another function of Hsc70 is as an ATPase in the disassembly of clathrin-coated vesicles during transport of membrane components through the cell.[5][16] It works with auxilin to remove clathrin from coated vesicles. In neurons, synaptojanin is also an important protein involved in vesicle uncoating.[5] Hsc70 is a key component of chaperone-mediated autophagy wherein it imparts selectivity to the proteins being degraded by this lysosomal pathway.[5][16]
Hsc70 vs Hsp70 comparison
[edit]Human Hsc70 has 85% identity with human Hsp70 (SDSC workbench, blosom26 default analysis). The scientific community has long assumed that Hsp70 and Hsc70 have similar cellular roles, but this assumption proved incomplete. While Hsc70 also performed chaperone functions under normal conditions, unlike canonical heat shock proteins, Hsc70 is constitutively expressed and performs functions related to normal cellular processes, such as protein ubiquitylation and degradation.[16][17]
Clinical significance
[edit]The Hsp70 member proteins are important apoptotic constituents. During a normal embryologic processes, or during cell injury (such as ischemia-reperfusion injury during heart attacks and strokes) or during developments and processes in cancer, an apoptotic cell undergoes structural changes including cell shrinkage, plasma membrane blebbing, nuclear condensation, and fragmentation of the DNA and nucleus. This is followed by fragmentation into apoptotic bodies that are quickly removed by phagocytes, thereby preventing an inflammatory response.[18] It is a mode of cell death defined by characteristic morphological, biochemical and molecular changes. It was first described as a "shrinkage necrosis", and then this term was replaced by apoptosis to emphasize its role opposite mitosis in tissue kinetics. In later stages of apoptosis the entire cell becomes fragmented, forming a number of plasma membrane-bounded apoptotic bodies which contain nuclear and or cytoplasmic elements. The ultrastructural appearance of necrosis is quite different, the main features being mitochondrial swelling, plasma membrane breakdown and cellular disintegration. Apoptosis occurs in many physiological and pathological processes. It plays an important role during embryonal development as programmed cell death and accompanies a variety of normal involutional processes in which it serves as a mechanism to remove "unwanted" cells.
Hsp70 member proteins, including Hsp72, inhibit apoptosis by acting on the caspase-dependent pathway and against apoptosis-inducing agents such as tumor necrosis factor-α (TNFα), staurosporine, and doxorubicin. This role leads to its involvement in many pathological processes, such as oncogenesis, neurodegeneration, and senescence. In particular, overexpression of HSP72 has been linked to the development some cancers, such as hepatocellular carcinoma, gastric cancers, colon cancers, breast cancers, and lung cancers, which led to its use as a prognostic marker for these cancers.[7] Elevated Hsp70 levels in tumor cells may increase malignancy and resistance to therapy by complexing, and hence, stabilizing, oncofetal proteins and products and transporting them into intracellular sites, thereby promoting tumor cell proliferation.[19][7] As a result, tumor vaccine strategies for Hsp70s have been highly successful in animal models and progressed to clinical trials.[7] One treatment, a Hsp72/AFP recombined vaccine, elicited robust protective immunity against AFP-expressing tumors in mice experiments. Therefore, the vaccine holds promise for treating hepatocellular carcinoma.[7] Alternatively, overexpression of Hsp70 can mitigate damage from ischemia-reperfusion in cardiac muscle, as well damage from neurodegenerative diseases, such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and spinocerebellar ataxias, and aging and cell senescence, as observed in centenarians subjected to heat shock challenge.[19][20] In particular, Hsc70 plays a protective role in the aforementioned diseases, as well as in other neuropsychiatric disorders such as schizophrenia.[21] Its protective role was further highlighted in a study that identified HSPA8 alongside other HSP70 proteins in a core sub-network of the wider chaperome interactome that functions as a proteostasis safeguard and that is repressed in aging brains and in the brains of Alzheimer's, Parkinson's and Huntington's disease patients.[22]
Interactions
[edit]Hsc70 forms a chaperone complex by interacting with the heat shock protein of 40 kDa (Hsp40), the heat shock protein of 90 kDa (Hsp90), the hsc70-interacting protein (HIP), the hsc70-hsp90 organizing protein (HOP), and the Bcl2-associated athanogene 1 protein (BAG1).[12]
HSPA8 has also been shown to interact with:
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000109971 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000015656 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d e f g "Entrez Gene: HSPA8 heat shock 70kDa protein 8".
- ^ a b c d Mayer MP, Bukau B (Mar 2005). "Hsp70 chaperones: cellular functions and molecular mechanism". Cellular and Molecular Life Sciences. 62 (6): 670–684. doi:10.1007/s00018-004-4464-6. PMC 2773841. PMID 15770419.
- ^ a b c d e f g Wang X, Wang Q, Lin H, Li S, Sun L, Yang Y (Feb 2013). "HSP72 and gp96 in gastroenterological cancers". Clinica Chimica Acta; International Journal of Clinical Chemistry. 417: 73–9. doi:10.1016/j.cca.2012.12.017. PMID 23266770.
- ^ Xilouri M, Stefanis L (Dec 2016). "Chaperone Mediated Autophagy: Starve to Prosper". Ageing Research Reviews. 32: 13–21. doi:10.1016/j.arr.2016.07.001. PMID 27484893. S2CID 884595.
- ^ Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C, Kroemer G (September 2001). "Heat-shock protein 70 antagonizes apoptosis-inducing factor". Nat. Cell Biol. 3 (9): 839–43. doi:10.1038/ncb0901-839. PMID 11533664. S2CID 21164493.
- ^ Zhang B, Rong R, Li H, Peng X, Xiong L, Wang Y, Yu X, Mao H (2015). "Heat shock protein 72 suppresses apoptosis by increasing the stability of X-linked inhibitor of apoptosis protein in renal ischemia/reperfusion injury". Mol Med Rep. 11 (3): 1793–9. doi:10.3892/mmr.2014.2939. PMC 4270332. PMID 25394481.
- ^ a b Zhang P, Leu JI, Murphy ME, George DL, Marmorstein R (2014). "Crystal structure of the stress-inducible human heat shock protein 70 substrate-binding domain in complex with peptide substrate". PLOS ONE. 9 (7): e103518. Bibcode:2014PLoSO...9j3518Z. doi:10.1371/journal.pone.0103518. PMC 4110032. PMID 25058147.
- ^ a b Majeski AE, Dice JF (2004). "Mechanisms of chaperone-mediated autophagy". Int. J. Biochem. Cell Biol. 36 (12): 2435–44. doi:10.1016/j.biocel.2004.02.013. PMID 15325583.
- ^ a b c Xie W, Zhang L, Jiao H, Guan L, Zha J, Li X, Wu M, Wang Z, Han J, You H (Jul 2015). "Chaperone-mediated autophagy prevents apoptosis by degrading BBC3/PUMA". Autophagy. 11 (9): 1623–1635. doi:10.1080/15548627.2015.1075688. PMC 4590652. PMID 26212789.
- ^ Diehl JA, Yang W, Rimerman RA, Xiao H, Emili A (March 2003). "Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase". Molecular and Cellular Biology. 23 (5): 1764–74. doi:10.1128/mcb.23.5.1764-1774.2003. PMC 151693. PMID 12588994.
- ^ Hatakeyama T, Dai P, Harada Y, Hino H, Tsukahara F, Maru Y, Otsuji E, Takamatsu T (2013). "Connexin43 functions as a novel interacting partner of heat shock cognate protein 70". Scientific Reports. 3: 2719. Bibcode:2013NatSR...3E2719H. doi:10.1038/srep02719. PMC 3779846. PMID 24056538.
- ^ a b c Goldfarb SB, Kashlan OB, Watkins JN, Suaud L, Yan W, Kleyman TR, Rubenstein RC (Apr 2006). "Differential effects of Hsc70 and Hsp70 on the intracellular trafficking and functional expression of epithelial sodium channels". Proceedings of the National Academy of Sciences of the United States of America. 103 (15): 5817–22. Bibcode:2006PNAS..103.5817G. doi:10.1073/pnas.0507903103. PMC 1458656. PMID 16585520.
- ^ Soss SE, Rose KL, Hill S, Jouan S, Chazin WJ (2015). "Biochemical and Proteomic Analysis of Ubiquitination of Hsc70 and Hsp70 by the E3 Ligase CHIP". PLOS ONE. 10 (5): e0128240. Bibcode:2015PLoSO..1028240S. doi:10.1371/journal.pone.0128240. PMC 4444009. PMID 26010904.
- ^ Kerr JF, Wyllie AH, Currie AR (Aug 1972). "Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics". British Journal of Cancer. 26 (4): 239–57. doi:10.1038/bjc.1972.33. PMC 2008650. PMID 4561027.
- ^ a b Mayer MP, Bukau B (Mar 2005). "Hsp70 chaperones: cellular functions and molecular mechanism". Cellular and Molecular Life Sciences. 62 (6): 670–84. doi:10.1007/s00018-004-4464-6. PMC 2773841. PMID 15770419.
- ^ Henstridge DC, Whitham M, Febbraio MA (2014). "Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes". Mol Metab. 3 (8): 781–93. doi:10.1016/j.molmet.2014.08.003. PMC 4216407. PMID 25379403.
- ^ Bozidis P, Hyphantis T, Mantas C, Sotiropoulou M, Antypa N, Andreoulakis E, Serretti A, Mavreas V, Antoniou K (Apr 2014). "HSP70 polymorphisms in first psychotic episode drug-naïve schizophrenic patients". Life Sciences. 100 (2): 133–7. doi:10.1016/j.lfs.2014.02.006. PMID 24548631.
- ^ Brehme M, Voisine C, Rolland T, Wachi S, Soper JH, Zhu Y, Orton K, Villella A, Garza D, Vidal M, Ge H, Morimoto RI (2014). "A conserved chaperome sub-network safeguards protein homeostasis in aging and neurodegenerative disease". Cell Rep. 9 (3): 1135–1150. doi:10.1016/j.celrep.2014.09.042. PMC 4255334. PMID 25437566.
- ^ a b c Takayama S, Xie Z, Reed JC (Jan 1999). "An evolutionarily conserved family of Hsp70/Hsc70 molecular chaperone regulators". The Journal of Biological Chemistry. 274 (2): 781–6. doi:10.1074/jbc.274.2.781. PMID 9873016.
- ^ Takayama S, Bimston DN, Matsuzawa S, Freeman BC, Aime-Sempe C, Xie Z, Morimoto RI, Reed JC (Aug 1997). "BAG-1 modulates the chaperone activity of Hsp70/Hsc70". The EMBO Journal. 16 (16): 4887–96. doi:10.1093/emboj/16.16.4887. PMC 1170124. PMID 9305631.
- ^ Miki K, Eddy EM (Apr 2002). "Tumor necrosis factor receptor 1 is an ATPase regulated by silencer of death domain". Molecular and Cellular Biology. 22 (8): 2536–43. doi:10.1128/MCB.22.8.2536-2543.2002. PMC 133739. PMID 11909948.
- ^ Ajuh P, Kuster B, Panov K, Zomerdijk JC, Mann M, Lamond AI (Dec 2000). "Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry". The EMBO Journal. 19 (23): 6569–81. doi:10.1093/emboj/19.23.6569. PMC 305846. PMID 11101529.
- ^ Yahata T, de Caestecker MP, Lechleider RJ, Andriole S, Roberts AB, Isselbacher KJ, Shioda T (Mar 2000). "The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors". The Journal of Biological Chemistry. 275 (12): 8825–34. doi:10.1074/jbc.275.12.8825. PMID 10722728.
- ^ a b Hatakeyama T, Dai P, Harada Y, Hino H, Tsukahara F, Maru Y, Otsuji E, Takamatsu T (2013). "Connexin43 functions as a novel interacting partner of heat shock cognate protein 70". Scientific Reports. 3: 2719. Bibcode:2013NatSR...3E2719H. doi:10.1038/srep02719. PMC 3779846. PMID 24056538.
- ^ Sarkar S, Pollack BP, Lin KT, Kotenko SV, Cook JR, Lewis A, Pestka S (Dec 2001). "hTid-1, a human DnaJ protein, modulates the interferon signaling pathway". The Journal of Biological Chemistry. 276 (52): 49034–42. doi:10.1074/jbc.M103683200. PMID 11679576.
- ^ Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, Klitgord N, Simon C, Boxem M, Milstein S, Rosenberg J, Goldberg DS, Zhang LV, Wong SL, Franklin G, Li S, Albala JS, Lim J, Fraughton C, Llamosas E, Cevik S, Bex C, Lamesch P, Sikorski RS, Vandenhaute J, Zoghbi HY, Smolyar A, Bosak S, Sequerra R, Doucette-Stamm L, Cusick ME, Hill DE, Roth FP, Vidal M (Oct 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- ^ Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (Sep 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070. S2CID 8235923.
- ^ Imai Y, Soda M, Hatakeyama S, Akagi T, Hashikawa T, Nakayama KI, Takahashi R (Jul 2002). "CHIP is associated with Parkin, a gene responsible for familial Parkinson's disease, and enhances its ubiquitin ligase activity". Molecular Cell. 10 (1): 55–67. doi:10.1016/S1097-2765(02)00583-X. PMID 12150907.
- ^ Ballinger CA, Connell P, Wu Y, Hu Z, Thompson LJ, Yin LY, Patterson C (Jun 1999). "Identification of CHIP, a novel tetratricopeptide repeat-containing protein that interacts with heat shock proteins and negatively regulates chaperone functions". Molecular and Cellular Biology. 19 (6): 4535–45. doi:10.1128/mcb.19.6.4535. PMC 104411. PMID 10330192.
Further reading
[edit]- Kiang JG (Dec 2004). "Inducible heat shock protein 70 kD and inducible nitric oxide synthase in hemorrhage/resuscitation-induced injury". Cell Research. 14 (6): 450–9. doi:10.1038/sj.cr.7290247. PMID 15625011. S2CID 21654486.
- Rasmussen HH, van Damme J, Puype M, Gesser B, Celis JE, Vandekerckhove J (Dec 1992). "Microsequences of 145 proteins recorded in the two-dimensional gel protein database of normal human epidermal keratinocytes". Electrophoresis. 13 (12): 960–9. doi:10.1002/elps.11501301199. PMID 1286667. S2CID 41855774.
- Hattori H, Liu YC, Tohnai I, Ueda M, Kaneda T, Kobayashi T, Tanabe K, Ohtsuka K (Feb 1992). "Intracellular localization and partial amino acid sequence of a stress-inducible 40-kDa protein in HeLa cells". Cell Structure and Function. 17 (1): 77–86. doi:10.1247/csf.17.77. PMID 1586970. S2CID 21909960.
- DeLuca-Flaherty C, McKay DB, Parham P, Hill BL (Sep 1990). "Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis". Cell. 62 (5): 875–87. doi:10.1016/0092-8674(90)90263-E. PMID 1975516. S2CID 9501568.
- Lim MY, Davis N, Zhang JY, Bose HR (Mar 1990). "The v-rel oncogene product is complexed with cellular proteins including its proto-oncogene product and heat shock protein 70". Virology. 175 (1): 149–60. doi:10.1016/0042-6822(90)90195-W. PMID 2155506.
- Welch WJ, Mizzen LA (Apr 1988). "Characterization of the thermotolerant cell. II. Effects on the intracellular distribution of heat-shock protein 70, intermediate filaments, and small nuclear ribonucleoprotein complexes". The Journal of Cell Biology. 106 (4): 1117–30. doi:10.1083/jcb.106.4.1117. PMC 2115010. PMID 2966179.
- Dworniczak B, Mirault ME (Jul 1987). "Structure and expression of a human gene coding for a 71 kd heat shock 'cognate' protein". Nucleic Acids Research. 15 (13): 5181–97. doi:10.1093/nar/15.13.5181. PMC 305955. PMID 3037489.
- Rensing SA, Maier UG (Jul 1994). "Phylogenetic analysis of the stress-70 protein family". Journal of Molecular Evolution. 39 (1): 80–6. Bibcode:1994JMolE..39...80R. doi:10.1007/BF00178252. PMID 7545947. S2CID 37505045.
- Lain B, Iriarte A, Mattingly JR, Moreno JI, Martinez-Carrion M (Oct 1995). "Structural features of the precursor to mitochondrial aspartate aminotransferase responsible for binding to hsp70". The Journal of Biological Chemistry. 270 (42): 24732–9. doi:10.1074/jbc.270.42.24732. PMID 7559589.
- Benaroudj N, Batelier G, Triniolles F, Ladjimi MM (Nov 1995). "Self-association of the molecular chaperone HSC70". Biochemistry. 34 (46): 15282–90. doi:10.1021/bi00046a037. PMID 7578144.
- Nunes SL, Calderwood SK (Aug 1995). "Heat shock factor-1 and the heat shock cognate 70 protein associate in high molecular weight complexes in the cytoplasm of NIH-3T3 cells". Biochemical and Biophysical Research Communications. 213 (1): 1–6. doi:10.1006/bbrc.1995.2090. PMID 7639722.
- Inoue A, Torigoe T, Sogahata K, Kamiguchi K, Takahashi S, Sawada Y, Saijo M, Taya Y, Ishii S, Sato N, Kikuchi K (Sep 1995). "70-kDa heat shock cognate protein interacts directly with the N-terminal region of the retinoblastoma gene product pRb. Identification of a novel region of pRb-mediating protein interaction". The Journal of Biological Chemistry. 270 (38): 22571–6. doi:10.1074/jbc.270.38.22571. PMID 7673249.
- Abe T, Konishi T, Hirano T, Kasai H, Shimizu K, Kashimura M, Higashi K (Jan 1995). "Possible correlation between DNA damage induced by hydrogen peroxide and translocation of heat shock 70 protein into the nucleus". Biochemical and Biophysical Research Communications. 206 (2): 548–55. doi:10.1006/bbrc.1995.1078. PMID 7826371.
- Furlini G, Vignoli M, Re MC, Gibellini D, Ramazzotti E, Zauli G, La Placa M (Jan 1994). "Human immunodeficiency virus type 1 interaction with the membrane of CD4+ cells induces the synthesis and nuclear translocation of 70K heat shock protein". The Journal of General Virology. 75 (1): 193–9. doi:10.1099/0022-1317-75-1-193. PMID 7906708.
- Maruyama K, Sugano S (Jan 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Tavaria M, Gabriele T, Anderson RL, Mirault ME, Baker E, Sutherland G, Kola I (Sep 1995). "Localization of the gene encoding the human heat shock cognate protein, HSP73, to chromosome 11". Genomics. 29 (1): 266–8. doi:10.1006/geno.1995.1242. PMID 8530083.
- Gao B, Eisenberg E, Greene L (Jul 1996). "Effect of constitutive 70-kDa heat shock protein polymerization on its interaction with protein substrate". The Journal of Biological Chemistry. 271 (28): 16792–7. doi:10.1074/jbc.271.28.16792. PMID 8663341.
- Egerton M, Moritz RL, Druker B, Kelso A, Simpson RJ (Jul 1996). "Identification of the 70kD heat shock cognate protein (Hsc70) and alpha-actinin-1 as novel phosphotyrosine-containing proteins in T lymphocytes". Biochemical and Biophysical Research Communications. 224 (3): 666–74. doi:10.1006/bbrc.1996.1082. PMID 8713105.
- Lamian V, Small GM, Feldherr CM (Oct 1996). "Evidence for the existence of a novel mechanism for the nuclear import of Hsc70". Experimental Cell Research. 228 (1): 84–91. doi:10.1006/excr.1996.0302. PMID 8892974.
- Hansen S, Midgley CA, Lane DP, Freeman BC, Morimoto RI, Hupp TR (Nov 1996). "Modification of two distinct COOH-terminal domains is required for murine p53 activation by bacterial Hsp70". The Journal of Biological Chemistry. 271 (48): 30922–8. doi:10.1074/jbc.271.48.30922. PMID 8940078.
External links
[edit]- Hsc70+Protein at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Heat shock cognate 71 kDa protein