Immune checkpoint



Immune checkpoints are regulators of the immune system. These pathways are crucial for self-tolerance, which prevents the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.[1]
Inhibitory checkpoint molecules are targets for cancer immunotherapy due to their potential for use in multiple types of cancers. Currently approved checkpoint inhibitors block CTLA4, PD-1 and PD-L1. For the related basic science discoveries, James P. Allison and Tasuku Honjo won the Tang Prize in Biopharmaceutical Science and the Nobel Prize in Physiology or Medicine in 2018.[2][3]
Stimulatory checkpoint molecules
[edit]Four stimulatory checkpoint molecules are members of the tumor necrosis factor (TNF) receptor superfamily—CD27, CD40, OX40, GITR and CD137. Another two stimulatory checkpoint molecules belong to the B7-CD28 superfamily—CD28 itself and ICOS.
- CD27: This molecule supports antigen-specific expansion of naïve T cells and is vital for the generation of T cell memory.[4] CD27 is also a memory marker of B cells.[5] CD27's activity is governed by the transient availability of its ligand, CD70, on lymphocytes and dendritic cells.[6] CD27 costimulation is known to suppress Th17 effector cell function.[7] The American biotech company Celldex Therapeutics is working on CDX-1127, an agonistic anti-CD27 monoclonal antibody[8] which in animal models has been shown to be effective in the context of T cell receptor stimulation.[9]
- CD28: This molecule is constitutively expressed on almost all human CD4+ T cells and on around half of all CD8 T cells. Binding with its two ligands are CD80 and CD86, expressed on dendritic cells, prompts T cell expansion. CD28 was the target of the TGN1412 'superagonist' which caused severe inflammatory reactions in the first-in-man study in London in March 2006.[10]
- CD40: This molecule, found on a variety of immune system cells including antigen presenting cells has CD40L, otherwise known as CD154 and transiently expressed on the surface of activated CD4+ T cells, as its ligand. CD40 signaling is known to ‘license’ dendritic cells to mature and thereby trigger T-cell activation and differentiation.[11] A now-defunct Seattle-based biotechnology company called VLST in-licensed an anti-CD40 agonist monoclonal antibody from Pfizer in 2012. The Swiss pharmaceutical company Roche acquired this project when VLST was shut down in 2013.[12]
- CD122: This molecule, which is the Interleukin-2 receptor beta sub-unit, is known to increase proliferation of CD8+ effector T cells.[13] The American biotechnology company Nektar Therapeutics is working on NKTR-214, a CD122-biased immune-stimulatory cytokine[14] Phase I results announced in Nov 2016.[15]
- CD137: When this molecule, also called 4-1BB, is bound by CD137 ligand, the result is T-cell proliferation. CD137-mediated signaling is also known to protect T cells, and in particular, CD8+ T cells from activation-induced cell death.[16] The German biotech company Pieris Pharmaceuticals has developed an engineered lipocalin that is bi-specific for CD137 and HER2.[17]
- OX40: This molecule, also called CD134, has OX40L, or CD252, as its ligand. Like CD27, OX40 promotes the expansion of effector and memory T cells, however it is also noted for its ability to suppress the differentiation and activity of T-regulatory cells, and also for its regulation of cytokine production.[18] OX40's value as a drug target primarily lies in the fact that, being transiently expressed after T-cell receptor engagement, it is only upregulated on the most recently antigen-activated T cells within inflammatory lesions.[19] Anti-OX40 monoclonal antibodies have been shown to have clinical utility in advanced cancer.[20] The pharma company AstraZeneca has three drugs in development targeting OX40: MEDI0562 is a humanised OX40 agonist; MEDI6469, murine OX4 agonist; and MEDI6383, an OX40 agonist[21]
- GITR: short for Glucocorticoid-Induced TNFR family Related gene, prompts T cell expansion, including Treg expansion.[22] The ligand for GITR is mainly expressed on antigen presenting cells.[23] Antibodies to GITR have been shown to promote an anti-tumor response through loss of Treg lineage stability.[24] The biotech company TG Therapeutics is working on anti-GITR antibodies[25]
- ICOS: This molecule, short for Inducible T-cell costimulator, and also called CD278, is expressed on activated T cells. Its ligand is ICOSL, expressed mainly on B cells and dendritic cells. The molecule seems to be important in T cell effector function.[26] The American biotechnology company Jounce Therapeutics is developing an ICOS agonist.
Inhibitory checkpoint molecules
[edit]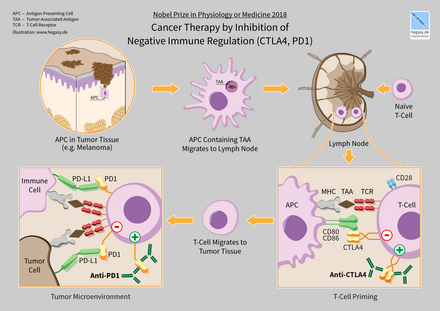
- A2AR & A2BR: The Adenosine A2A receptor is regarded as an important checkpoint in cancer therapy because adenosine in the immune microenvironment, leading to the activation of the A2a receptor, is negative immune feedback loop and the tumor microenvironment has relatively high concentrations of adenosine.[27] Recently, the role of the A2B receptor in the immune system has been demonstrated.[28][29] Studies propose that in tissues where they are co-expressed, A2A and A2B form dimers under the majority control of the A2B receptor.[30]
- B7-H3: also called CD276, was originally understood to be a co-stimulatory molecule[31] but is now regarded as co-inhibitory.[32] The American biotechnology company MacroGenics is working on MGA271 is an Fc-optimized monoclonal antibody that targets B7-H3.[33] B7-H3’s receptors have not yet been identified.[34]
- B7-H4: also called VTCN1, is expressed by tumor cells and tumor-associated macrophages and plays a role in tumour escape.[35]
- BTLA: This molecule, short for B and T Lymphocyte Attenuator and also called CD272, has HVEM (Herpesvirus Entry Mediator) as its ligand. Surface expression of BTLA is gradually downregulated during differentiation of human CD8+ T cells from the naive to effector cell phenotype, however tumor-specific human CD8+ T cells express high levels of BTLA.[36]
- CTLA-4: short for Cytotoxic T-Lymphocyte-Associated protein 4 and also called CD152, is the target of Bristol-Myers Squibb's melanoma drug Yervoy, which gained FDA approval in March 2011. Expression of CTLA-4 on Treg cells serves to control T cell proliferation.[37][38]
- IDO: short for Indoleamine 2,3-dioxygenase, is a tryptophan catabolic enzyme with immune-inhibitory properties. Another important molecule is TDO, tryptophan 2,3-dioxygenase. IDO is known to suppress T and NK cells, generate and activate Tregs and myeloid-derived suppressor cells, and promote tumour angiogenesis.[39][38]
- KIR: short for Killer-cell Immunoglobulin-like Receptor, is a receptor for MHC Class I molecules on Natural Killer cells. Bristol-Myers Squibb is working on Lirilumab, a monoclonal antibody to KIR.
- LAG3: short for Lymphocyte Activation Gene-3, works to suppress an immune response by action to Tregs[40] as well as direct effects on CD8+ T cells.[41][38] Bristol-Myers Squibb is in Phase I with an anti-LAG3 monoclonal antibody called BMS-986016.[42]
- NOX2: short for nicotinamide adenine dinucleotide phosphate NADPH oxidase isoform 2, is an enzyme of myeloid cells that generates immunosuppressive reactive oxygen species. Genetic and pharmacological inhibition of NOX2 in myeloid cells improves anti-tumor functions of adjacent NK cells and T cells and also triggers autoimmunity in humans and experimental animals. NOX2 is the target of Ceplene that has gained approval for use in acute myeloid leukemia within the EU. [43][38]
- PD-1: short for Programmed Death 1 (PD-1) receptor, has two ligands, PD-L1 and PD-L2. This checkpoint is the target of Merck & Co.'s melanoma drug Keytruda, which gained FDA approval in September 2014. The checkpoint is also the target of EMD Serono (Merck KGaA)'s drug Bavencio, which gained FDA approval in 2017. An advantage of targeting PD-1 is that it can restore immune function in the tumor microenvironment.[44][38]
- TIM-3: short for T-cell Immunoglobulin domain and Mucin domain 3, expresses on activated human CD4+ T cells and regulates Th1 and Th17 cytokines.[45] TIM-3 acts as a negative regulator of Th1/Tc1 function by triggering cell death upon interaction with its ligand, galectin-9.[46][38]
- VISTA: Short for V-domain Ig suppressor of T cell activation, VISTA is primarily expressed on hematopoietic cells[47] so that consistent expression of VISTA on leukocytes within tumors may allow VISTA blockade to be effective across a broad range of solid tumors.[48][38]
- SIGLEC7 (Sialic acid-binding immunoglobulin-type lectin 7, also designated as CD328) and SIGLEC9 (Sialic acid-binding immunoglobulin-type lectin 9, also designated as CD329) are proteins found on the surface of various immune cells, including natural killer cells and macrophages (SIGLEC7) and neutrophils, macrophages, dendritic cells and activated T-cells (SIGLEC9).[49] SIGLECs 7 and 9 suppress the immune function of these cells by binding to terminal sialic acid on glycans that cover the surface of cells.[50][51]
Immune checkpoint inhibitors
[edit]Drugs or drug candidates that inhibit/block the inhibitory checkpoint molecules are sometimes known as checkpoint inhibitors; this idea is often referred to as immune checkpoint blockade, or simply checkpoint blockade.[52][38] Checkpoint inhibitor drugs have seen growth in pharmaceutical research in cancer by companies including Bristol-Myers Squibb, Merck, Merck KGaA, Roche and AstraZeneca.[53]
References
[edit]- ^ Pardoll DM (March 2012). "The blockade of immune checkpoints in cancer immunotherapy". Nature Reviews. Cancer. 12 (4): 252–264. doi:10.1038/nrc3239. PMC 4856023. PMID 22437870.
- ^ "2014 Tang Prize in Biopharmaceutical Science". Archived from the original on 2017-10-20. Retrieved 2016-06-18.
- ^ Devlin H (2018-10-01). "James P Allison and Tasuku Honjo win Nobel prize for medicine". the Guardian. Retrieved 2018-10-01.
- ^ Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J (November 2000). "CD27 is required for generation and long-term maintenance of T cell immunity". Nature Immunology. 1 (5): 433–440. doi:10.1038/80877. PMID 11062504. S2CID 36108378.
- ^ Agematsu K (April 2000). "Memory B cells and CD27". Histology and Histopathology. 15 (2): 573–576. doi:10.14670/HH-15.573. PMID 10809378.
- ^ Borst J, Hendriks J, Xiao Y (June 2005). "CD27 and CD70 in T cell and B cell activation". Current Opinion in Immunology. 17 (3): 275–281. doi:10.1016/j.coi.2005.04.004. PMID 15886117.
- ^ Coquet JM, Middendorp S, van der Horst G, Kind J, Veraar EA, Xiao Y, et al. (January 2013). "The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity". Immunity. 38 (1): 53–65. doi:10.1016/j.immuni.2012.09.009. PMID 23159439.
- ^ "CDX-1127 – Monoclonal Antibody Targeting CD27". Celldex Therapeutics. Archived from the original on 2018-10-03. Retrieved 2015-06-04.
- ^ He LZ, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, et al. (October 2013). "Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice". Journal of Immunology. 191 (8): 4174–4183. doi:10.4049/jimmunol.1300409. PMID 24026078.
- ^ Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, et al. (October 2010). "Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells". British Journal of Pharmacology. 161 (3): 512–526. doi:10.1111/j.1476-5381.2010.00922.x. PMC 2990151. PMID 20880392.
- ^ O'Sullivan B, Thomas R (January 1, 2003). "CD40 and dendritic cell function". Critical Reviews in Immunology. 23 (1–2): 83–107. doi:10.1615/critrevimmunol.v23.i12.50. PMID 12906261.
- ^ Zimm A (April 12, 2014). "Cancer 'Miracle' Patients Studied Anew for Disease Clues". Bloomberg. Retrieved 25 June 2015.
- ^ Boyman O, Sprent J (February 2012). "The role of interleukin-2 during homeostasis and activation of the immune system". Nature Reviews. Immunology. 12 (3): 180–190. doi:10.1038/nri3156. PMID 22343569. S2CID 22680847.
- ^ "Nektar and MD Anderson Cancer Center Announce Phase 1/2 Clinical Research Collaboration for NKTR-214, a CD122-Biased Immuno-Stimulatory Cytokine". Nektar Therapeutics. June 2, 2015. Archived from the original on 19 December 2017. Retrieved 25 June 2015.
- ^ Immunotherapy, NKTR-214, Shows Activity Against Solid Tumors in Clinical Trial
- ^ Singh R, Kim YH, Lee SJ, Eom HS, Choi BK (Feb 2024). "4-1BB immunotherapy: advances and hurdles". Experimental & Molecular Medicine volume. 56 (1): 32–39. doi:10.1038/s12276-023-01136-4. PMC 10834507. PMID 38172595.
- ^ "Pieris Pharmaceuticals to present data on novel anti-CD137 and HER2 bispecific immuno-oncology program at UBS Global Healthcare Conference". Pieris Pharmaceuticals. 19 May 2015. Archived from the original on 3 October 2018. Retrieved 5 June 2015.
- ^ Croft M, So T, Duan W, Soroosh P (May 2009). "The significance of OX40 and OX40L to T-cell biology and immune disease". Immunological Reviews. 229 (1): 173–191. doi:10.1111/j.1600-065x.2009.00766.x. PMC 2729757. PMID 19426222.
- ^ Weinberg AD, Morris NP, Kovacsovics-Bankowski M, Urba WJ, Curti BD (November 2011). "Science gone translational: the OX40 agonist story". Immunological Reviews. 244 (1): 218–231. doi:10.1111/j.1600-065x.2011.01069.x. PMC 3622727. PMID 22017441.
- ^ Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, et al. (December 2013). "OX40 is a potent immune-stimulating target in late-stage cancer patients". Cancer Research. 73 (24): 7189–7198. doi:10.1158/0008-5472.can-12-4174. PMC 3922072. PMID 24177180.
- ^ "Q1 2015 Result". AstraZeneca. 24 April 2015. Retrieved 5 June 2015.
- ^ Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, et al. (March 2004). "GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations". European Journal of Immunology. 34 (3): 613–622. doi:10.1002/eji.200324804. PMID 14991590.
- ^ Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C (May 2007). "GITR/GITRL: more than an effector T cell co-stimulatory system". European Journal of Immunology. 37 (5): 1165–1169. doi:10.1002/eji.200636933. PMID 17407102.
- ^ Schaer DA, Budhu S, Liu C, Bryson C, Malandro N, Cohen A, et al. (November 2013). "GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability". Cancer Immunology Research. 1 (5): 320–331. doi:10.1158/2326-6066.cir-13-0086. PMC 3885345. PMID 24416730.
- ^ "TG Therapeutics Enters Into a Global Collaboration With Checkpoint Therapeutics to Develop and Commercialize Novel Immuno-Oncology Targeted Antibodies". TG Therapeutics. 4 March 2015. Retrieved 5 June 2015.
- ^ Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, et al. (January 2008). "ICOS controls the pool size of effector-memory and regulatory T cells". Journal of Immunology. 180 (2): 774–782. doi:10.4049/jimmunol.180.2.774. PMID 18178815.
- ^ Leone RD, Lo YC, Powell JD (April 8, 2015). "A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy". Computational and Structural Biotechnology Journal. 13: 265–272. doi:10.1016/j.csbj.2015.03.008. PMC 4415113. PMID 25941561.
- ^ Tay AH, Prieto-Díaz R, Neo S, Tong L, Chen X, Carannante V, et al. (May 2022). "A2B adenosine receptor antagonists rescue lymphocyte activity in adenosine-producing patient-derived cancer models". Journal for Immunotherapy of Cancer. 10 (5): e004592. doi:10.1136/jitc-2022-004592. PMC 9115112. PMID 35580926. S2CID 248858436.
- ^ Prieto-Díaz, Rubén; González-Gómez, Manuel; Fojo-Carballo, Hugo; Azuaje, Jhonny; El Maatougui, Abdelaziz; Majellaro, Maria; Loza, María I.; Brea, José; Fernández-Dueñas, Víctor; Paleo, M. Rita; Díaz-Holguín, Alejandro; Garcia-Pinel, Beatriz; Mallo-Abreu, Ana; Estévez, Juan C.; Andújar-Arias, Antonio (2022-12-14). "Exploring the Effect of Halogenation in a Series of Potent and Selective A 2B Adenosine Receptor Antagonists". Journal of Medicinal Chemistry. 66 (1): 890–912. doi:10.1021/acs.jmedchem.2c01768. ISSN 0022-2623. PMC 9841532. PMID 36517209. S2CID 254759241.
- ^ Hinz S, Navarro G, Borroto-Escuela D, Seibt BF, Ammon YC, de Filippo E, et al. (March 2018). "Adenosine A2A receptor ligand recognition and signaling is blocked by A2B receptors". Oncotarget. 9 (17): 13593–13611. doi:10.18632/oncotarget.24423. PMC 5862601. PMID 29568380.
- ^ Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, et al. (March 2001). "B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production". Nature Immunology. 2 (3): 269–274. doi:10.1038/85339. PMID 11224528. S2CID 43480199.
- ^ Leitner J, Klauser C, Pickl WF, Stöckl J, Majdic O, Bardet AF, et al. (July 2009). "B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction". European Journal of Immunology. 39 (7): 1754–1764. doi:10.1002/eji.200839028. PMC 2978551. PMID 19544488.
- ^ "MacroGenics Provides Update on Corporate Progress and First Quarter 2015 Financial Results". MacroGenics. 6 May 2015. Retrieved 5 June 2015.
- ^ Mao Y, Li W, Chen K, Xie Y, Liu Q, Yao M, et al. (February 2015). "B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer". Oncotarget. 6 (5): 3452–3461. doi:10.18632/oncotarget.3097. PMC 4413666. PMID 25609202.
- ^ Dangaj D, Lanitis E, Zhao A, Joshi S, Cheng Y, Sandaltzopoulos R, et al. (August 2013). "Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses". Cancer Research. 73 (15): 4820–4829. doi:10.1158/0008-5472.can-12-3457. PMC 3732560. PMID 23722540.
- ^ Derré L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, et al. (January 2010). "BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination". The Journal of Clinical Investigation. 120 (1): 157–167. doi:10.1172/jci40070. PMC 2799219. PMID 20038811.

- ^ Kolar P, Knieke K, Hegel JK, Quandt D, Burmester GR, Hoff H, Brunner-Weinzierl MC (January 2009). "CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice". Arthritis and Rheumatism. 60 (1): 123–132. doi:10.1002/art.24181. PMID 19116935.
- ^ a b c d e f g h Syn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet. Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- ^ Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ (July 2014). "Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer". Cancer Immunology, Immunotherapy. 63 (7): 721–735. doi:10.1007/s00262-014-1549-4. PMC 4384696. PMID 24711084.
- ^ Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. (October 2004). "Role of LAG-3 in regulatory T cells". Immunity. 21 (4): 503–513. doi:10.1016/j.immuni.2004.08.010. PMID 15485628.
- ^ Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, et al. (November 2007). "LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems". The Journal of Clinical Investigation. 117 (11): 3383–3392. doi:10.1172/jci31184. PMC 2000807. PMID 17932562.
- ^ Clinical trial number NCT01968109 for "Safety Study of Anti-LAG-3 With and Without Anti-PD-1 in the Treatment of Solid Tumors" at ClinicalTrials.gov
- ^ Martner A, Aydin E, Hellstrand K (February 2019). "NOX2 in autoimmunity, tumor growth and metastasis". The Journal of Pathology. 247 (2): 151–154. doi:10.1002/path.5175. PMC 6587556. PMID 30270440.
- ^ Philips GK, Atkins M (January 2015). "Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies". International Immunology. 27 (1): 39–46. doi:10.1093/intimm/dxu095. PMID 25323844.
- ^ Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, et al. (September 2009). "TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines". European Journal of Immunology. 39 (9): 2492–2501. doi:10.1002/eji.200939274. PMC 2759376. PMID 19676072.
- ^ Zhu C, Anderson AC, Kuchroo VK (August 11, 2010). "TIM-3 and its regulatory role in immune responses". Current Topics in Microbiology and Immunology. 350: 1–15. doi:10.1007/82_2010_84. ISBN 978-3-642-19544-0. PMID 20700701.
- ^ Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y, et al. (March 2011). "VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses". The Journal of Experimental Medicine. 208 (3): 577–592. doi:10.1084/jem.20100619. PMC 3058578. PMID 21383057.
- ^ Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O'Connell S, et al. (April 2014). "VISTA is an immune checkpoint molecule for human T cells". Cancer Research. 74 (7): 1924–1932. doi:10.1158/0008-5472.CAN-13-1504. PMC 3979527. PMID 24691993.
- ^ Stanczak MA, Siddiqui SS, Trefny MP, Thommen DS, Boligan KF, von Gunten S, et al. (November 2018). "Self-associated molecular patterns mediate cancer immune evasion by engaging Siglecs on T cells". The Journal of Clinical Investigation. 128 (11): 4912–4923. doi:10.1172/JCI120612. PMC 6205408. PMID 30130255.
- ^ Crocker PR, Paulson JC, Varki A (April 2007). "Siglecs and their roles in the immune system". Nature Reviews. Immunology. 7 (4): 255–266. doi:10.1038/nri2056. PMID 17380156. S2CID 26722878.
- ^ Macauley MS, Crocker PR, Paulson JC (October 2014). "Siglec-mediated regulation of immune cell function in disease". Nature Reviews. Immunology. 14 (10): 653–666. doi:10.1038/nri3737. PMC 4191907. PMID 25234143.
- ^ Pardoll DM (March 2012). "The blockade of immune checkpoints in cancer immunotherapy". Nature Reviews. Cancer. 12 (4): 252–264. doi:10.1038/nrc3239. PMC 4856023. PMID 22437870.
- ^ "Cancer drugs are getting better and dearer". The Economist. 2017-05-04. Retrieved 2017-05-19.