d5SICS

 | |
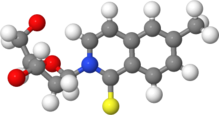 | |
| Names | |
|---|---|
| IUPAC name 2-(2-Deoxy-β-D-erythro-pentofuranosyl)-6-methylisoquinoline-1(2H)-thione | |
| Systematic IUPAC name 2-[(2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)oxolan-2-yl]-6-methylisoquinoline-1(2H)-thione | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H17NO3S | |
| Molar mass | 291.37 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
d5SICS is an artificial nucleoside containing 6-methylisoquinoline-1-thione-2-yl group instead of a base.
It pairs up with dNaM in a hydrophobic interaction. It was not able to be removed by the error-correcting machinery of the E. coli into which it was inserted.[1][2] The pairing of d5SICS–dNaM is mediated by packing and hydrophobic forces instead of hydrogen bonding, which occurs in natural base pairs. Therefore, in free DNA, rings of d5SICS and dNaM are placed in parallel planes instead of the same plane.[3] The d5SICS-dNaM pairing replaced an older dNaM-dTPT3 pairing.[4]
References
[edit]- ^ "Bacterium survives unnatural DNA transplant". Rsc.org. Retrieved July 29, 2015.
- ^ Malyshev, D. A. (2012). "Efficient and sequence-independent replication of DNA containing a third base pair establishes a functional six-letter genetic alphabet". Proceedings of the National Academy of Sciences. 109 (30): 12005–12010. Bibcode:2012PNAS..10912005M. doi:10.1073/pnas.1205176109. PMC 3409741. PMID 22773812.
- ^ Betz, Karin; et al. (2013). "Structural Insights into DNA Replication Without Hydrogen-Bonds". J Am Chem Soc. 135 (49): 18637–43. doi:10.1021/ja409609j. PMC 3982147. PMID 24283923.
- ^ Ben Guarino (24 January 2017). "Biologists breed life form with lab-made DNA. Don't call it 'Jurassic Park.'". Washington Post.