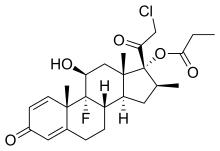
Clobetasol propionate

 | |
| Clinical data | |
|---|---|
| Pronunciation | /kloʊˈbeɪtəsɒl/[1] |
| Trade names | Dermovate, Temovate, Clovate, others |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.042.380 |
| Chemical and physical data | |
| Formula | C25H32ClFO5 |
| Molar mass | 466.97 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clobetasol propionate is a corticosteroid used to treat skin conditions such as eczema, contact dermatitis, seborrheic dermatitis, steroid responsive dermatosis, and psoriasis (including scalp and plaque-type).[3][4][5]
It is applied to the skin as a cream, foam, gel, liquid, solution, ointment, or shampoo.[4][6][5] Use should be short term and only if other weaker corticosteroids are not effective.[6] Use is not recommended in rosacea or perioral dermatitis.[4]
Common side effects include skin irritation, dry skin, redness, pimples, and telangiectasia.[4] Serious side effects may include adrenal suppression, allergic reactions, cellulitis, and Cushing's syndrome.[4] Use in pregnancy and breastfeeding is of unclear safety.[7] Clobetasol is believed to work by activating steroid receptors.[4]
Clobetasol propionate was patented in 1968 and came into medical use in 1978.[8] It is available as a generic medication.[6] In 2022, it was the 156th most commonly prescribed medication in the United States, with more than 3 million prescriptions.[9][10]
Medical uses
[edit]Clobetasol propionate is used for the treatment of various skin disorders including eczema, herpes labialis,[11] psoriasis, and lichen sclerosus. It is also used to treat several auto-immune diseases including alopecia areata, lichen planus (auto immune skin nodules), and mycosis fungoides (T-cell skin lymphoma). It is used as first-line treatment for both acute and chronic GVHD of the skin.[12]
Clobetasol propionate is used cosmetically for skin whitening, although this use is controversial. The U.S. Food and Drug Administration has not approved it for that purpose, and sales without a prescription are illegal in the U.S. Nonetheless, skin-whitening creams containing this ingredient can sometimes be found in beauty supply stores in New York City and on the internet. It is also sold internationally, and does not require a prescription in some countries. Whitening creams with clobetasol propionate, such as Hyprogel, can make skin thin and easily bruised, with visible capillaries, and acne. It can also lead to hypertension, elevated blood sugar, suppression of the body's natural steroids, and stretch marks, which may be permanent.[13]
Clobetasol propionate is, along with mercury and hydroquinone, "amongst the most toxic, the latter in higher concentrations, and most used agents in lightening products." Many products sold illegally have higher concentrations of clobetasol propionate than is permitted for prescription drugs.[14]
Clobetasol 0.05% is considered a member of the "Super-high potency" (Group 1) group of topical corticosteroids [5] out of 7 total groups in the United States classification system of topical corticosteroids. The weaker strength formulation of 0.025% belongs to Group 2 (“High Potency”).
Society and culture
[edit]Brand names
[edit]Clobetasol propionate is marketed and sold worldwide under numerous names, including Clobex, Clob-x (Colombia), Clovate, Clobet (Biolab Thailand) Clonovate (T.O. Chemicals, Thailand), Cormax (Watson, US), Haloderm (Switzerland, by ELKO Org), Pentasol (Colombia), Cosvate, Clop (Cadila Healthcare, India), Propysalic (India), Temovate (US), Dermovate[15] (GlaxoSmithKline, Canada, Estonia, Pakistan, Switzerland, Ukraine, Portugal, Romania, Israel), Olux, ClobaDerm, Tenovate, Dermatovate (Brazil, Mexico), Butavate, Movate, Novate, Salac (Argentina), and Powercort, Lotasbat and Kloderma (Indonesia), Lemonvate and Clobesol (Italy), Dovate (South Africa), Delor (Ethiopia), Psovate (Turkey) or Skineal (Nigeria).
References
[edit]- ^ "Clobetasol Propionate Topical Ointment 0.05% Information". Drug Encyclopedia. Kaiser Permanente. Archived from the original on 28 August 2021. Retrieved 9 October 2009.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ "DailyMed: Betasol dipropionate". NIH.gov.
- ^ a b c d e f "Clobetasol Propionate Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 28 August 2021. Retrieved 13 April 2019.
- ^ a b c "Clobetasol (topical): Drug information". UpToDate. 2024.
- ^ a b c British National Formulary: BNF 76 (76th ed.). Pharmaceutical Press. 2018. p. 1210. ISBN 978-0-85711-338-2.
- ^ "Clobetasol topical Use During Pregnancy". Drugs.com. Archived from the original on 28 August 2021. Retrieved 13 April 2019.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 487. ISBN 9783527607495. Archived from the original on 28 November 2023. Retrieved 3 September 2020.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- ^ "Clobetasol Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- ^ Hull C, McKeough M, Sebastian K, Kriesel J, Spruance S (March 2009). "Valacyclovir and topical clobetasol gel for the episodic treatment of herpes labialis: a patient-initiated, double-blind, placebo-controlled pilot trial". Journal of the European Academy of Dermatology and Venereology. 23 (3): 263–7. doi:10.1111/j.1468-3083.2008.03047.x. PMID 19143902. S2CID 205588376.
- ^ E. Fougera and Co. "Clobetasol Propionate Cream Usp, 0.05% Clobetasol Propionate Ointment USP, 0.05%". Daily Med. U.S. National Library of Medicine. Archived from the original on 23 May 2009. Retrieved 3 July 2009.
- ^ Saint Louis C (15 January 2010). "Creams Offering Lighter Skin May Bring Risks". New York Times. Archived from the original on 19 February 2017. Retrieved 24 February 2017.
- ^ Gbetoh MH, Amyot M (October 2016). "Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada". Environmental Research. 150: 403–410. Bibcode:2016ER....150..403G. doi:10.1016/j.envres.2016.06.030. hdl:1866/19006. PMID 27372064.
- ^ "Dermovate 0.05% W/V Ointment – Clobetasol Topical(0.05% W/V)". Glaxo SmithKline Pharmaceuticals Ltd. GNHIndia.com. Archived from the original on 16 July 2021. Retrieved 16 July 2021.