Glial cell line-derived neurotrophic factor

Glial cell line-derived neurotrophic factor (GDNF) is a protein that, in humans, is encoded by the GDNF gene.[5] GDNF is a small protein that potently promotes the survival of many types of neurons.[6] It signals through GFRα receptors, particularly GFRα1. It is also responsible for the determination of spermatogonia into primary spermatocytes, i.e. it is received by RET proto-oncogene (RET) and by forming gradient with SCF it divides the spermatogonia into two cells. As the result there is retention of spermatogonia and formation of spermatocyte.[7][full citation needed]
GDNF family of ligands (GFL)
[edit]GDNF was discovered in 1991,[8] and is the first member of the GDNF family of ligands (GFL) found.
Function
[edit]GDNF is highly distributed throughout both the peripheral and central nervous system. It can be secreted by astrocytes, oligodendrocytes, Schwann cells, motor neurons, and skeletal muscle during the development and growth of neurons and other peripheral cells.[9]
The GDNF gene encodes a highly conserved neurotrophic factor. The recombinant form of this protein was shown to promote the survival and differentiation of dopaminergic neurons in culture, and was able to prevent apoptosis of motor neurons induced by axotomy. GDNF is synthesized as a 211 amino acid-long protein precursor, pro-GDNF.[9] The pre-sequence leads the protein to the endoplasmic reticulum for secretion. While secretion takes place, the protein precursor folds via a sulfide-sulfide bond and dimerizes. The protein then is modified by N-linked glycosylation during packaging and preparation in the Golgi apparatus. Finally, the protein precursor undergoes proteolysis due to a proteolytic consensus sequence in its C-terminus end and is cleaved to 134 amino acids.[9] Proteases that play a role in the proteolysis of pro-GDNF into mature GDNF include furin, PACE4, PC5A, PC5B, and PC7. Because multiple proteases can cleave the protein precursor, four different mature forms of GDNF can be produced.[9] The proteolytic processing of GDNF requires SorLA, a protein sorting receptor. SorLA does not bind to any other GFLs.[10] The mature form of the protein is a ligand for the product of the RET (rearranged during transfection) protooncogene. In addition to the transcript encoding GDNF, two additional alternative transcripts encoding distinct proteins, referred to as astrocyte-derived trophic factors, have also been described. Mutations in this gene may be associated with Hirschsprung's disease.[6]
GDNF has the ability to activate the ERK-1 and ERK-2 isoforms of MAP kinase in sympathetic neurons as well as P13K/AKT pathways via activation of its receptor tyrosine kinases.[11][12] It can also activate Src-family kinases through its GFRα1 receptor.[13]
The most prominent feature of GDNF is its ability to support the survival of dopaminergic[14] and motor neurons.[citation needed] It prevents apoptosis in motor neurons during development, decreases the overall loss of neurons during development, rescues cells from axotomy-induced death, and prevents chronic degeneration.[9]
These neuronal populations die in the course of Parkinson's disease and amyotrophic lateral sclerosis (ALS). GDNF also regulates kidney development and spermatogenesis, and has a powerful and rapid negative (ameliorating) effect on alcohol consumption.[15] GDNF also promotes hair follicle formation and cutaneous wound healing by targeting resident hair follicle stem cells (BSCs) in the bulge compartment.[16]
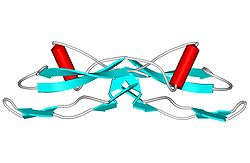
Structure
[edit]GDNF has a structure that is similar to TGF beta 2.[11] GDNF has two finger-like structures that interact with the GFRα1 receptor. N-linked glycosylation, which occurs during the secretion of pro-GDNF, takes place at the tip of one of the finger-like structures. The C-terminal of mature GDNF plays an important role in binding with both Ret and the GFRα1 receptor. The C-terminus forms a loop out of the interactions between cysteines Cys131, Cy133, Cys68, and Cys 72.[9]
Interactions
[edit]Glial cell line-derived neurotrophic factor has been shown to interact with GFRA1[9][17] and GDNF family receptor alpha 1. The activity of GDNF, as well as other GFLs, is mediated by RET receptor tyrosine kinase. In order for the receptor to modulate GDNF activity, GDNF must also be bound to GFRα1.[11] The intensity and duration of RET signaling can likewise be monitored by the GPI-anchor of GFRα1 by interacting with compartments of the cell membrane, such as lipid rafts or cleavage by phospholipases.[12] In cells that lack RET, some GDNF family ligand members also have the ability to be activated through the neural cell adhesion molecule (NCAM). GDNF can associate with NCAM through its GFRα1 GPI-anchor. The association between GDNF and NCAM results in the activation of cytoplasmic protein tyrosine kinases Fyn and FAK.[18]
Potential as therapeutics
[edit]GDNF has been investigated as a treatment for Parkinson's disease, though early research has not shown a significant effect.[8][19] Vitamin D potently induces GDNF expression.[20]
In 2012, the University of Bristol began a five-year clinical trial on Parkinson's sufferers, in which surgeons introduced a port into the skull of each of the 41 participants through which the drug could be delivered, in order to enable it to reach the damaged cells directly.[21] The results of the double-blind trial, where half the participants were randomly assigned to receive regular infusions of GDNF and the other half placebo infusions, did not show a statistically significant difference between the active treatment group and those who received placebo, but did confirm the effects on damaged brain cells.[22]
The study was funded by Parkinson’s UK (Grant J-1102), with support from The Cure Parkinson’s Trust (whose founder, Tom Isaacs, was one of the participants[23]) and was sponsored by North Bristol NHS Trust. Study drug, additional project resources and supplementary funding was provided by MedGenesis Therapeutix Inc., who in turn received program funding support from the Michael J. Fox Foundation for Parkinson’s Research. Renishaw plc manufactured the CED device on behalf of North Bristol NHS Trust and provided additional technical and analytical support. The Gatsby Foundation provided a 3T MRI scanner.[24]
Neuropsychopharmacology
[edit]Administration of the African hallucinogen ibogaine potently increases GDNF expression in the ventral tegmental area, which is the mechanism behind the alkaloid's anti-addictive effect.[25] Rodent models for a non-psychedelic analogue of this compound show promise in promoting GDNF expression without the hallucinogenic or cardiotoxic effects well documented for ibogaine.[26]
There is evidence, that Gdnf is an alcohol-responsive gene upregulated during short-term alcohol intake but downregulated during withdrawal from excessive alcohol intake.[27] Specifically, one study showed that alcohol withdrawal alters the expression of Gdnf in addiction related brain areas like the ventral tegmental area (VTA) and the Nucleus Accumbens as well as DNA methylation of the Gdnf gene in rats.[28]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000168621 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000022144 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (May 1993). "GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons". Science. 260 (5111): 1130–2. Bibcode:1993Sci...260.1130L. doi:10.1126/science.8493557. PMID 8493557.
- ^ a b "Entrez Gene: GDNF glial cell derived neurotrophic factor". Archived from the original on 2010-03-07. Retrieved 2017-08-31.
- ^ Scott F. Gilbert
- ^ a b Vastag B (August 2010). "Biotechnology: Crossing the barrier". Nature. 466 (7309): 916–8. doi:10.1038/466916a. PMID 20725015.
- ^ a b c d e f g Cintrón-Colón AF, Almeida-Alves G, Boynton AM, Spitsbergen JM (October 2020). "GDNF synthesis, signaling, and retrograde transport in motor neurons". Cell and Tissue Research. 382 (1): 47–56. doi:10.1007/s00441-020-03287-6. PMC 7529617. PMID 32897420.
- ^ Glerup S, Lume M, Olsen D, Nyengaard JR, Vaegter CB, Gustafsen C, et al. (January 2013). "SorLA controls neurotrophic activity by sorting of GDNF and its receptors GFRα1 and RET". Cell Reports. 3 (1): 186–99. doi:10.1016/j.celrep.2012.12.011. PMID 23333276.
- ^ a b c Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, et al. (December 1996). "Neurturin, a relative of glial-cell-line-derived neurotrophic factor". Nature. 384 (6608): 467–70. Bibcode:1996Natur.384..467K. doi:10.1038/384467a0. PMID 8945474. S2CID 4238843.
- ^ a b Ibáñez CF, Andressoo JO (January 2017). "Biology of GDNF and its receptors - Relevance for disorders of the central nervous system". Neurobiology of Disease. 97 (Pt B): 80–89. doi:10.1016/j.nbd.2016.01.021. PMID 26829643. S2CID 17588722.
- ^ Airaksinen MS, Saarma M (May 2002). "The GDNF family: signalling, biological functions and therapeutic value". Nature Reviews. Neuroscience. 3 (5): 383–94. doi:10.1038/nrn812. PMID 11988777. S2CID 2480120.
- ^ Oo TF, Kholodilov N, Burke RE (June 2003). "Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal glial cell line-derived neurotrophic factor in vivo". The Journal of Neuroscience. 23 (12): 5141–8. doi:10.1523/JNEUROSCI.23-12-05141.2003. PMC 6741204. PMID 12832538.
- ^ Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D (June 2008). "GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse". Proceedings of the National Academy of Sciences of the United States of America. 105 (23): 8114–9. Bibcode:2008PNAS..105.8114C. doi:10.1073/pnas.0711755105. PMC 2423415. PMID 18541917.
- ^ Lisse TS, Sharma M, Vishlaghi N, Pullagura SR, Braun RE (Jun 2020). "GDNF promotes hair formation and cutaneous wound healing by targeting bulge stem cells". npj Regenerative Medicine. 5 (13): 13. doi:10.1038/s41536-020-0098-z. PMC 7293257. PMID 32566252.
- ^ Cik M, Masure S, Lesage AS, Van Der Linden I, Van Gompel P, Pangalos MN, et al. (September 2000). "Binding of GDNF and neurturin to human GDNF family receptor alpha 1 and 2. Influence of cRET and cooperative interactions". The Journal of Biological Chemistry. 275 (36): 27505–12. doi:10.1074/jbc.M000306200. PMID 10829012.
- ^ Paratcha G, Ledda F, Ibáñez CF (June 2003). "The neural cell adhesion molecule NCAM is an alternative signaling receptor for GDNF family ligands". Cell. 113 (7): 867–79. doi:10.1016/s0092-8674(03)00435-5. PMID 12837245.
- ^ "Intermittent Bilateral Intraputamenal Treatment with GDNF". The Michael J. Fox Foundation for Parkinson's Research | Parkinson's Disease.
- ^ Eserian JK (July 2013). "Vitamin D as an effective treatment approach for drug abuse and addiction". Journal of Medical Hypotheses and Ideas. 7 (2): 35–39. doi:10.1016/j.jmhi.2013.02.001.
Vitamin D is a potent inducer of endogenous GDNF. The most prominent feature of GDNF is its ability to support the survival of dopaminergic neurons.
- ^ "The radical drug trial hoping for a miracle Parkinson's cure". BBC News. Archived from the original on 10 March 2019. Retrieved 10 March 2019.
- ^ "GDNF clinical trial offers hope of restoring brain cells damaged in Parkinson's". Parkinsons UK. 27 February 2019. Archived from the original on 27 March 2019. Retrieved 10 March 2019.
- ^ "Pioneering trial offers hope for restoring brain cells damaged in Parkinson's". University of Bristol. 2019-02-19. Archived from the original on 2019-03-27. Retrieved 2019-03-27.
- ^ Whone A, Luz M, Boca M, Woolley M, Mooney L, Dharia S, et al. (March 2019). "Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson's disease". Brain. 142 (3): 512–525. doi:10.1093/brain/awz023. PMC 6391602. PMID 30808022.
- ^ He DY, McGough NN, Ravindranathan A, Jeanblanc J, Logrip ML, Phamluong K, et al. (January 2005). "Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption". The Journal of Neuroscience. 25 (3): 619–28. doi:10.1523/JNEUROSCI.3959-04.2005. PMC 1193648. PMID 15659598.
- ^ Cameron LP, Tombari RJ, Lu J, Pell AJ, Hurley ZQ, Ehinger Y, et al. (January 2021). "A non-hallucinogenic psychedelic analogue with therapeutic potential". Nature. 589 (7842): 474–479. Bibcode:2021Natur.589..474C. doi:10.1038/s41586-020-3008-z. PMC 7874389. PMID 33299186.
- ^ Barak S, Ahmadiantehrani S, Logrip ML, Ron D (May 2019). "GDNF and alcohol use disorder". Addiction Biology. 24 (3): 335–343. doi:10.1111/adb.12628. PMC 6215739. PMID 29726054.
- ^ Maier HB, Neyazi M, Neyazi A, Hillemacher T, Pathak H, Rhein M, et al. (February 2020). "Alcohol consumption alters Gdnf promoter methylation and expression in rats". Journal of Psychiatric Research. 121: 1–9. doi:10.1016/j.jpsychires.2019.10.020. PMID 31710958. S2CID 207964134.
Further reading
[edit]- Hofstra RM, Osinga J, Buys CH (1998). "Mutations in Hirschsprung disease: when does a mutation contribute to the phenotype". European Journal of Human Genetics. 5 (4): 180–5. doi:10.1159/000484760. PMID 9359036.
- Martucciello G, Ceccherini I, Lerone M, Jasonni V (July 2000). "Pathogenesis of Hirschsprung's disease". Journal of Pediatric Surgery. 35 (7): 1017–25. doi:10.1053/jpsu.2000.7763. PMID 10917288.
- Schindelhauer D, Schuffenhauer S, Gasser T, Steinkasserer A, Meitinger T (August 1995). "The gene coding for glial cell line derived neurotrophic factor (GDNF) maps to chromosome 5p12-p13.1". Genomics. 28 (3): 605–7. doi:10.1006/geno.1995.1202. PMID 7490108.
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, et al. (January 1995). "Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo". Nature. 373 (6512): 335–9. Bibcode:1995Natur.373..335T. doi:10.1038/373335a0. PMID 7830766. S2CID 4340992.
- Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, et al. (January 1995). "Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF". Nature. 373 (6512): 344–6. Bibcode:1995Natur.373..344O. doi:10.1038/373344a0. PMID 7830769. S2CID 2863274.
- Schaar DG, Sieber BA, Sherwood AC, Dean D, Mendoza G, Ramakrishnan L, et al. (December 1994). "Multiple astrocyte transcripts encode nigral trophic factors in rat and human". Experimental Neurology. 130 (2): 387–93. doi:10.1006/exnr.1994.1218. PMID 7867768. S2CID 37574956.
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (May 1993). "GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons". Science. 260 (5111): 1130–2. Bibcode:1993Sci...260.1130L. doi:10.1126/science.8493557. PMID 8493557.
- Bermingham N, Hillermann R, Gilmour F, Martin JE, Fisher EM (December 1995). "Human glial cell line-derived neurotrophic factor (GDNF) maps to chromosome 5". Human Genetics. 96 (6): 671–3. doi:10.1007/BF00210297. PMID 8522325. S2CID 30960307.
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, et al. (March 1996). "Functional recovery in parkinsonian monkeys treated with GDNF". Nature. 380 (6571): 252–5. Bibcode:1996Natur.380..252G. doi:10.1038/380252a0. PMID 8637574. S2CID 4313985.
- Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, et al. (June 1996). "GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF". Cell. 85 (7): 1113–24. doi:10.1016/S0092-8674(00)81311-2. PMID 8674117. S2CID 1724567.
- Angrist M, Bolk S, Halushka M, Lapchak PA, Chakravarti A (November 1996). "Germline mutations in glial cell line-derived neurotrophic factor (GDNF) and RET in a Hirschsprung disease patient". Nature Genetics. 14 (3): 341–4. doi:10.1038/ng1196-341. PMID 8896568. S2CID 24350470.
- Salomon R, Attié T, Pelet A, Bidaud C, Eng C, Amiel J, et al. (November 1996). "Germline mutations of the RET ligand GDNF are not sufficient to cause Hirschsprung disease". Nature Genetics. 14 (3): 345–7. doi:10.1038/ng1196-345. PMID 8896569. S2CID 22375940.
- Ivanchuk SM, Myers SM, Eng C, Mulligan LM (December 1996). "De novo mutation of GDNF, ligand for the RET/GDNFR-alpha receptor complex, in Hirschsprung disease". Human Molecular Genetics. 5 (12): 2023–6. doi:10.1093/hmg/5.12.2023. PMID 8968758.
- Haniu M, Hui J, Young Y, Le J, Katta V, Lee R, et al. (December 1996). "Glial cell line-derived neurotrophic factor: selective reduction of the intermolecular disulfide linkage and characterization of its disulfide structure". Biochemistry. 35 (51): 16799–805. doi:10.1021/bi9605550. PMID 8988018.
- Bär KJ, Facer P, Williams NS, Tam PK, Anand P (April 1997). "Glial-derived neurotrophic factor in human adult and fetal intestine and in Hirschsprung's disease". Gastroenterology. 112 (4): 1381–5. doi:10.1016/S0016-5085(97)70154-9. PMID 9098026.
- Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T, et al. (December 1997). "GFRalpha-2 and GFRalpha-3 are two new receptors for ligands of the GDNF family". The Journal of Biological Chemistry. 272 (52): 33111–7. doi:10.1074/jbc.272.52.33111. PMID 9407096.
- Eng C, Myers SM, Kogon MD, Sanicola M, Hession C, Cate RL, et al. (February 1998). "Genomic structure and chromosomal localization of the human GDNFR-alpha gene". Oncogene. 16 (5): 597–601. doi:10.1038/sj.onc.1201573. PMID 9482105.
- Amiel J, Salomon R, Attié T, Pelet A, Trang H, Mokhtari M, et al. (March 1998). "Mutations of the RET-GDNF signaling pathway in Ondine's curse". American Journal of Human Genetics. 62 (3): 715–7. doi:10.1086/301759. PMC 1376953. PMID 9497256.
- Yamaguchi Y, Wada T, Suzuki F, Takagi T, Hasegawa J, Handa H (August 1998). "Casein kinase II interacts with the bZIP domains of several transcription factors". Nucleic Acids Research. 26 (16): 3854–61. doi:10.1093/nar/26.16.3854. PMC 147779. PMID 9685505.
- Oo TF, Kholodilov N, Burke RE (June 2003). "Regulation of natural cell death in dopaminergic neurons of the substantia nigra by striatal glial cell line-derived neurotrophic factor in vivo". The Journal of Neuroscience. 23 (12): 5141–8. doi:10.1523/JNEUROSCI.23-12-05141.2003. PMC 6741204. PMID 12832538.
External links
[edit]- Glial+Cell+Line-Derived+Neurotrophic+Factor at the U.S. National Library of Medicine Medical Subject Headings (MeSH)