Advanced glycation end-product

Advanced glycation end-products (AGEs) derive from a chemical reaction – the Maillard reaction – between reducing sugars, such as glucose, and amino compounds formed by food processing or cooking, and during digestion and long-term molecular interactions.[1][2] In vivo, glucose may irreversibly react with long-life proteins, DNA, and lipids in a process called glycation (the same as the Maillard reaction), which forms AGEs.[1][2] Over decades in humans, the glycation process may cause protein and lipid aberrations, possibly involved in normal aging effects and development of glycation- and aging-related diseases.[2][3]
In aging individuals, AGEs may be associated with the initiation of inflammatory substances causing further tissue damage and increased disease risk.[1][3][4]
AGEs are under preliminary research as possible biomarkers of aging and the development, or worsening, of chronic diseases, such as diabetes, atherosclerosis and Alzheimer's disease.[1][2][3][5] As of 2024, the evidence for AGEs having a pathological role in aging diseases is generally inconsistent or inconclusive, with no causal association demonstrated between foods, AGEs and diseases.[1]
Putative formation mechanism
[edit]A possible mechanism for formation of AGEs in vivo involves the reaction between an amino group of lysine and the carbonyl group of glucose, forming a Schiff base, which in turn forms an Amadori rearrangement.[1][2] Some Amadori products are converted to AGEs, and others form reactive carbonyl species, which may react with proteins to form AGEs.[1][2] In humans, histones in the cell nucleus are richest in lysine, and therefore form the glycated protein N(6)-carboxymethyllysine.[1][2]
A receptor nicknamed RAGE, from receptor for advanced glycation end products, is found on many cells, including endothelial cells, smooth muscle, cells of the immune system [which?] from tissue such as lung, liver, and kidney.[1][3] This receptor, when binding AGEs, is under preliminary research to determine if it contributes to age- and diabetes-related chronic inflammatory diseases.[1][2]
The pathogenesis of this process is hypothesized to activation of the nuclear factor kappa B (NF-κB) following AGE binding.[1] NF-κB controls several genes involved in inflammation.[6] AGEs can be detected and quantified using bioanalytical and immunological methods.[7]
Dietary sources
[edit]Animal-derived foods that are high in fat and protein are generally AGE-rich, and are prone to further AGE formation during cooking.[1][8] However, only low molecular weight AGEs are absorbed through diet, and vegetarians have been found to have higher concentrations of overall AGEs compared to non-vegetarians.[9]
Effects
[edit]
AGEs can be produced in the body and in manufactured foods.[1][2][3] The accumulation of AGEs may have causative roles in several age-related diseases by forming adducts with proteins and lipids.[1][2][3] In preliminary research, AGEs affect nearly every type of cell and molecule in the body, and may be a factor in aging[2][10] and some age-related chronic diseases.[1][3][11] They are also believed to play a causative role in the vascular complications of diabetes mellitus.[12]
AGEs may arise under certain pathological conditions, such as oxidative stress due to hyperglycemia in patients with diabetes.[1][3][13] AGEs may have a role as proinflammatory mediators in gestational diabetes.[14]
In the context of cardiovascular disease, a possible AGE mechanism is to induce crosslinking of collagen, which can cause vascular stiffening and entrapment of low-density lipoprotein particles (LDL) in the artery walls.[1] AGEs can also cause glycation of LDL which can promote its oxidation.[15] Oxidized LDL is one of the major factors in the development of atherosclerosis.[16] AGEs can bind to RAGE receptors and cause oxidative stress as well as activation of inflammatory pathways in vascular endothelial cells.[1][2][3]
In other diseases
[edit]AGEs have been implicated in Alzheimer's disease and cardiovascular diseases.[1][2][3]
According to in vitro research, the mechanism by which AGEs may induce damage is through a process called cross-linking that causes intracellular damage and apoptosis.[2][17]
Pathology
[edit]In laboratory studies, AGEs have a range of pathological effects, such as:[18][19]
- Increased vascular permeability
- Increased arterial stiffness
- Inhibition of vascular dilation by interfering with nitric oxide
- Oxidizing LDL
- Binding cells—including macrophage, endothelial, and mesangial—to induce the secretion of a variety of cytokines
- Enhanced oxidative stress
- Hemoglobin-AGE levels are elevated in diabetic individuals.[20] Therefore, substances that inhibit AGE formation may limit the progression of disease and may offer new tools for therapeutic interventions in the therapy of AGE-mediated disease[21][22]
- AGEs have specific cellular receptors; the best-characterized are those called RAGE.[2] The activation of cellular RAGE on endothelium, mononuclear phagocytes, and lymphocytes triggers the generation of free radicals and the expression of inflammatory gene mediators.[23] Such increases in oxidative stress lead to the activation of the transcription factor NF-κB and promote the expression of NF-κB regulated genes that have been associated with atherosclerosis.[21]
As of 2024, there is no conclusive clinical evidence for AGEs having a pathological role in aging diseases, and no causality has been demonstrated between processed foods, AGEs, and onset of aging or age-related diseases.[1]
Clearance
[edit]In clearance, or the rate at which a substance is removed or cleared from the body, it has been found that the cellular proteolysis of AGEs—the breakdown of proteins—produces AGE peptides and "AGE free adducts" (AGE adducts bound to single amino acids). These latter, after being released into the plasma, can be excreted in the urine.[24]
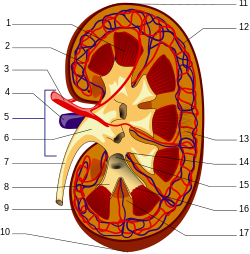
Nevertheless, the resistance of extracellular matrix proteins to proteolysis renders their advanced glycation end products less conducive to being eliminated.[24] While the AGE free adducts are released directly into the urine, AGE peptides are endocytosed by the epithelial cells of the proximal tubule and then degraded by the endolysosomal system to produce AGE amino acids. It is thought that these acids are then returned to the kidney's inside space, or lumen, for excretion. [18] AGE free adducts are the major form through which AGEs are excreted in urine, with AGE-peptides occurring to a lesser extent[18] but accumulating in the plasma of patients with chronic kidney failure.[24]
Larger, extracellularly derived AGE proteins cannot pass through the basement membrane of the renal corpuscle and must first be degraded into AGE peptides and AGE free adducts. Peripheral macrophage[18] as well as liver sinusoidal endothelial cells and Kupffer cells [25] have been implicated in this process, although the real-life involvement of the liver has been disputed. [26]

Large AGE proteins unable to enter the Bowman's capsule are capable of binding to receptors on endothelial and mesangial cells and to the mesangial matrix.[18] Activation of RAGE induces production of a variety of cytokines, including TNFβ, which mediates an inhibition of metalloproteinase and increases production of mesangial matrix, leading to glomerulosclerosis[19] and decreasing kidney function in patients with unusually high AGE levels.
Although the only form suitable for urinary excretion, the breakdown products of AGE — peptides and free adducts — are more aggressive than the AGE proteins from which they are derived, and they can perpetuate related pathology in people with diabetes, even after hyperglycemia has been brought under control.[18]
Research
[edit]Ongoing studies are performed to specify mechanisms that selectively inhibit the glycation process, and to understand how glycated molecules could be protected from further deterioration, possibly by manipulating the glyoxalase enzyme system to detoxify AGEs.[2]
Development of candidate drugs by the pharmaceutical industry includes compounds whose mechanism of action is to inhibit or revert the glycation process.[2]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t Hellwig M, Diel P, Eisenbrand G, et al. (September 2024). "Dietary glycation compounds - implications for human health". Critical Reviews in Toxicology. 54 (8): 485–617. doi:10.1080/10408444.2024.2362985. PMID 39150724.
- ^ a b c d e f g h i j k l m n o p q Uceda AB, Mariño L, Casasnovas R, et al. (April 2024). "An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition". Biophysical Reviews. 16 (2): 189–218. doi:10.1007/s12551-024-01188-4. PMC 11078917. PMID 38737201.
- ^ a b c d e f g h i j Rungratanawanich W, Qu Y, Wang X, et al. (February 2021). "Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury". Experimental and Molecular Medicine. 53 (2): 168–188. doi:10.1038/s12276-021-00561-7. PMC 8080618. PMID 33568752.
- ^ Flint B, Tadi P (4 January 2023). "Pathophysiology, Glycation. In: Physiology, Aging". StatPearls, US National Library of Medicine. Retrieved 27 June 2025.
- ^ Vistoli G, De Maddis, D, Cipak, A, et al. (August 2013). "Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation" (PDF). Free Radic Res. 47: Suppl 1:3–27. doi:10.3109/10715762.2013.815348. PMID 23767955. S2CID 207517855.
- ^ Liu T, Zhang L, Joo D, et al. (December 2017). "NF-κB signaling in inflammation". Signal Transduction and Targeted Therapy. 2 (1): 17023–. doi:10.1038/sigtrans.2017.23. PMC 5661633. PMID 29158945.
- ^ Ashraf JM, Ahmad S, Choi I, et al. (November 2015). "Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches: Technological Progress in Age Detection". IUBMB Life. 67 (12): 897–913. doi:10.1002/iub.1450. PMID 26597014.
- ^ Uribarri J, Woodruff S, Goodman S, et al. (June 2010). "Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet". Journal of the American Dietetic Association. 110 (6): 911–916.e12. doi:10.1016/j.jada.2010.03.018. PMC 3704564. PMID 20497781.
- ^ Poulsen MW, Hedegaard RV, Andersen JM, et al. (October 2013). "Advanced glycation endproducts in food and their effects on health". Food and Chemical Toxicology. 60: 10–37. doi:10.1016/j.fct.2013.06.052. PMID 23867544.
- ^ Chaudhuri J, Bains Y, Guha S, et al. (4 September 2018). "The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality". Cell Metabolism. 28 (3): 337–352. doi:10.1016/j.cmet.2018.08.014. PMC 6355252. PMID 30184484.
- ^ Glenn J, Stitt A (2009). "The role of advanced glycation end products in retinal ageing and disease". Biochimica et Biophysica Acta (BBA) - General Subjects. 1790 (10): 1109–1116. doi:10.1016/j.bbagen.2009.04.016. PMID 19409449.
- ^ Yan SF, D'Agati V, Schmidt AM, et al. (2007). "Receptor for Advanced Glycation Endproducts (RAGE): a formidable force in the pathogenesis of the cardiovascular complications of diabetes & aging". Current Molecular Medicine. 7 (8): 699–710. doi:10.2174/156652407783220732. PMID 18331228.
- ^ Brownlee M (June 2005). "The pathobiology of diabetic complications: a unifying mechanism". Diabetes. 54 (6): 1615–25. doi:10.2337/diabetes.54.6.1615. PMID 15919781.
- ^ Pertyńska-Marczewska M, Głowacka E, Sobczak M, et al. (11 January 2009). "Glycation Endproducts, Soluble Receptor for Advanced Glycation Endproducts and Cytokines in Diabetic and Non-diabetic Pregnancies". American Journal of Reproductive Immunology. 61 (2): 175–182. doi:10.1111/j.1600-0897.2008.00679.x. PMID 19143681. S2CID 3186554.
- ^ Prasad A, Bekker P, Tsimikas S (2012). "Advanced Glycation End Products and Diabetic Cardiovascular Disease". Cardiology in Review. 20 (4): 177–183. doi:10.1097/CRD.0b013e318244e57c. PMID 22314141. S2CID 8471652.
- ^ Di Marco E, Gray SP, Jandeleit-Dahm K (2013). "Diabetes Alters Activation and Repression of Pro- and Anti-Inflammatory Signaling Pathways in the Vasculature". Frontiers in Endocrinology. 4: 68. doi:10.3389/fendo.2013.00068. PMC 3672854. PMID 23761786.
- ^ Shaikh S, Nicholson LF (July 2008). "Advanced glycation end products induce in vitro cross-linking of α-synuclein and accelerate the process of intracellular inclusion body formation". Journal of Neuroscience Research. 86 (9): 2071–2082. doi:10.1002/jnr.21644. PMID 18335520. S2CID 37510479.
- ^ a b c d e f Gugliucci A, Bendayan M (1996). "Renal fate of circulating advanced glycated end products (AGE): evidence for reabsorption and catabolism of AGE peptides by renal proximal tubular cells". Diabetologia. 39 (2): 149–60. doi:10.1007/BF00403957. PMID 8635666.
- ^ a b Yan Hd, Li Xz, Xie Jm, et al. (May 2007). "Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells". Chinese Medical Journal. 120 (9): 787–793. doi:10.1097/00029330-200705010-00010. PMID 17531120.
- ^ Kostolanská J, Jakus V, Barák L (May 2009). "HbA1c and serum levels of advanced glycation and oxidation protein products in poorly and well controlled children and adolescents with type 1 diabetes mellitus". Journal of Pediatric Endocrinology & Metabolism. 22 (5): 433–42. doi:10.1515/JPEM.2009.22.5.433. PMID 19618662. S2CID 23150519.
- ^ a b Bierhaus A, Hofmann MA, Ziegler R, et al. (March 1998). "AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept". Cardiovascular Research. 37 (3): 586–600. doi:10.1016/S0008-6363(97)00233-2. PMID 9659442.
- ^ Thornalley, P.J. (1996). "Advanced glycation and the development of diabetic complications. Unifying the involvement of glucose, methylglyoxal and oxidative stress". Endocrinol. Metab. 3: 149–166.
- ^ Hofmann MA, Drury S, Fu C, et al. (June 1999). "RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides". Cell. 97 (7): 889–901. doi:10.1016/S0092-8674(00)80801-6. PMID 10399917. S2CID 7208198.
- ^ a b c Gugliucci A, Mehlhaff K, Kinugasa E, et al. (2007). "Paraoxonase-1 concentrations in end-stage renal disease patients increase after hemodialysis: correlation with low molecular AGE adduct clearance". Clin. Chim. Acta. 377 (1–2): 213–20. doi:10.1016/j.cca.2006.09.028. PMID 17118352.
- ^ Smedsrød B, Melkko J, Araki N, et al. (1997). "Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells". Biochem. J. 322 (Pt 2): 567–73. doi:10.1042/bj3220567. PMC 1218227. PMID 9065778.
- ^ Svistounov D, Smedsrød B (2004). "Hepatic clearance of advanced glycation end products (AGEs)—myth or truth?". J. Hepatol. 41 (6): 1038–40. doi:10.1016/j.jhep.2004.10.004. PMID 15582139.