Lead stearate

 | |
| Names | |
|---|---|
| Other names Lead(2+) octadecanoate, lead(II) stearate, lead distearate | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.012.733 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C 36H 70PbO 4 | |
| Molar mass | 774.14 |
| Appearance | White powder |
| Density | 1.4 g/cm3 |
| Melting point | 115.7 °C (240.3 °F; 388.8 K) |
| Boiling point | 359.4 °C (678.9 °F; 632.5 K) |
| Slightly soluble | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H302, H332, H360, H373 | |
| P260, P261, P281, P304, P340, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
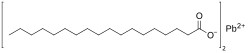
Lead stearate is a metal-organic compound, a salt of lead and stearic acid with the chemical formula C
36H
70PbO
4.[1] The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.[2] The compound is toxic.
Synthesis
[edit]The compound can be prepared by reacting stearic acid, lead(II) oxide, and a catalyst acetic acid.[3]
Also, an exchange reaction between lead(II) acetate and sodium stearate:
Physical properties
[edit]White powder with a slight fatty odor. Sinks in water.[4] Hygroscopic in air.
Slightly soluble in water.[1] Soluble in hot ethanol.
Uses
[edit]The compound is used as a drier in oil paints and varnishes to speed the polymerization and oxidation processes. Also used as a lubricant and stabilizer in vinyl polymers and as a corrosion inhibitor in petroleum products.[5][6][7]
References
[edit]- ^ a b "Lead Stearate". American Elements. Retrieved 7 March 2023.
- ^ "T3DB: Lead stearate". t3db.ca. Retrieved 7 March 2023.
- ^ "Preparation process of lead stearate based on melting method". 18 December 2013. Retrieved 7 March 2023.
- ^ "LEAD STEARATE | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 7 March 2023.
- ^ "Lead Stearate » Waldies Co. Ltd". Waldies Co. Ltd. Retrieved 7 March 2023.
- ^ Encyclopedia of Chemical Technology: Fuel resources to heat stabilizers. Wiley. 1991. p. 1074. ISBN 978-0-471-52669-8. Retrieved 7 March 2023.
- ^ Titow, M. V. (6 December 2012). PVC Technology. Springer Science & Business Media. p. 269. ISBN 978-94-009-5614-8. Retrieved 7 March 2023.

