Silicon

| Silicon | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | ||||||||||||||||||||||||||||||||||||
| Allotropes | see Allotropes of silicon | |||||||||||||||||||||||||||||||||||
| Appearance | crystalline, reflective with bluish-tinged faces | |||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Si) | ||||||||||||||||||||||||||||||||||||
| Silicon in the periodic table | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 14 | |||||||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | |||||||||||||||||||||||||||||||||||
| Period | period 3 | |||||||||||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p2 | |||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 4 | |||||||||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||||||||
| Melting point | 1687 K (1414 °C, 2577 °F) | |||||||||||||||||||||||||||||||||||
| Boiling point | 3538 K (3265 °C, 5909 °F) | |||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 2.329085 g/cm3[3] | |||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 2.57 g/cm3 | |||||||||||||||||||||||||||||||||||
| Heat of fusion | 50.21 kJ/mol | |||||||||||||||||||||||||||||||||||
| Heat of vaporization | 383 kJ/mol | |||||||||||||||||||||||||||||||||||
| Molar heat capacity | 19.789 J/(mol·K) | |||||||||||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||||||||
| Oxidation states | common: −4, +4 −3,[4] −2,[4] −1,[4] 0,[5] +1,[4][6] +2,[4] +3[4] | |||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | |||||||||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 111 pm | |||||||||||||||||||||||||||||||||||
| Covalent radius | 111 pm | |||||||||||||||||||||||||||||||||||
| Van der Waals radius | 210 pm | |||||||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||||||||
| Crystal structure | face-centered diamond-cubic (cF8) | |||||||||||||||||||||||||||||||||||
| Lattice constant | a = 543.0986 pm (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||
| Thermal expansion | 2.556×10−6/K (at 20 °C)[3] | |||||||||||||||||||||||||||||||||||
| Thermal conductivity | 149 W/(m⋅K) | |||||||||||||||||||||||||||||||||||
| Electrical resistivity | 2.3×103 Ω⋅m (at 20 °C)[7] | |||||||||||||||||||||||||||||||||||
| Band gap | 1.12 eV (at 300 K) | |||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[8] | |||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −3.9×10−6 cm3/mol (298 K)[9] | |||||||||||||||||||||||||||||||||||
| Young's modulus | 130–188 GPa[10] | |||||||||||||||||||||||||||||||||||
| Shear modulus | 51–80 GPa[10] | |||||||||||||||||||||||||||||||||||
| Bulk modulus | 97.6 GPa[10] | |||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 8433 m/s (at 20 °C) | |||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.064–0.28[10] | |||||||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | |||||||||||||||||||||||||||||||||||
| CAS Number | 7440-21-3 | |||||||||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||||||||
| Naming | after Latin silex or silicis, meaning 'flint' | |||||||||||||||||||||||||||||||||||
| Prediction | Antoine Lavoisier (1787) | |||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Jöns Jacob Berzelius[11][12] (1823) | |||||||||||||||||||||||||||||||||||
| Named by | Thomas Thomson (1817) | |||||||||||||||||||||||||||||||||||
| Isotopes of silicon | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table: carbon is above it; and germanium, tin, lead, and flerovium are below it. It is relatively unreactive. Silicon is a significant element that is essential for several physiological and metabolic processes in plants. Silicon is widely regarded as the predominant semiconductor material due to its versatile applications in various electrical devices such as transistors, solar cells, integrated circuits, and others. These may be due to its significant band gap, expansive optical transmission range, extensive absorption spectrum, surface roughening, and effective anti-reflection coating.[14]
Because of its high chemical affinity for oxygen, it was not until 1823 that Jöns Jakob Berzelius was first able to prepare it and characterize it in pure form. Its oxides form a family of anions known as silicates. Its melting and boiling points of 1414 °C and 3265 °C, respectively, are the second highest among all the metalloids and nonmetals, being surpassed only by boron.[a]
Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is widely distributed throughout space in cosmic dusts, planetoids, and planets as various forms of silicon dioxide (silica) or silicates. More than 90% of the Earth's crust is composed of silicate minerals, making silicon the second most abundant element in the Earth's crust (about 28% by mass), after oxygen.
Most silicon is used commercially without being separated, often with very little processing of the natural minerals. Such use includes industrial construction with clays, silica sand, and stone. Silicates are used in Portland cement for mortar and stucco, and mixed with silica sand and gravel to make concrete for walkways, foundations, and roads. They are also used in whiteware ceramics such as porcelain, and in traditional silicate-based soda–lime glass and many other specialty glasses. Silicon compounds such as silicon carbide are used as abrasives and components of high-strength ceramics. Silicon is the basis of the widely used synthetic polymers called silicones.
The late 20th century to early 21st century has been described as the Silicon Age (also known as the Digital Age or Information Age) because of the large impact that elemental silicon has on the modern world economy. The small portion of very highly purified elemental silicon used in semiconductor electronics (<15%) is essential to the transistors and integrated circuit chips used in most modern technology such as smartphones and other computers. In 2019, 32.4% of the semiconductor market segment was for networks and communications devices, and the semiconductors industry is projected to reach $726.73 billion by 2027.[15]
Silicon is an essential element in biology. Only traces are required by most animals, but some sea sponges and microorganisms, such as diatoms and radiolaria, secrete skeletal structures made of silica. Silica is deposited in many plant tissues.[16]
History
[edit]Owing to the abundance of silicon in the Earth's crust, natural silicon-based materials have been used for thousands of years. Silicon rock crystals were familiar to various ancient civilizations, such as the predynastic Egyptians who used it for beads and small vases, as well as the ancient Chinese. Glass containing silica was manufactured by the Egyptians since at least 1500 BC, as well as by the ancient Phoenicians. Natural silicate compounds were also used in various types of mortar for construction of early human dwellings.[17]
Discovery
[edit]
In 1787, Antoine Lavoisier suspected that silica might be an oxide of a fundamental chemical element,[18] but the chemical affinity of silicon for oxygen is high enough that he had no means to reduce the oxide and isolate the element.[19] After an attempt to isolate silicon in 1808, Sir Humphry Davy proposed the name "silicium" for silicon, from the Latin silex, silicis for flint, and adding the "-ium" ending because he believed it to be a metal.[20] Most other languages use transliterated forms of Davy's name, sometimes adapted to local phonology (e.g. German Silizium, Turkish silisyum, Catalan silici, Armenian Սիլիցիում or Silitzioum). A few others use instead a calque of the Latin root (e.g. Russian кремний, from кремень "flint"; Greek πυρίτιο from πυρ "fire"; Finnish pii from piikivi "flint", Czech křemík from křemen "quartz", "flint").[21]
Gay-Lussac and Thénard are thought to have prepared impure amorphous silicon in 1811, through the heating of recently isolated potassium metal with silicon tetrafluoride, but they did not purify and characterize the product, nor identify it as a new element.[22] Silicon was given its present name in 1817 by Scottish chemist Thomas Thomson. He retained part of Davy's name but added "-on" because he believed that silicon was a nonmetal similar to boron and carbon.[23] In 1824, Jöns Jacob Berzelius prepared amorphous silicon using approximately the same method as Gay-Lussac (reducing potassium fluorosilicate with molten potassium metal), but purifying the product to a brown powder by repeatedly washing it.[24] As a result, he is usually given credit for the element's discovery.[25][26] The same year, Berzelius became the first to prepare silicon tetrachloride; silicon tetrafluoride had already been prepared long before in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid.[19] In 1823 for the first time Jacob Berzelius discovered silicon tetrachloride (SiCl4).[27] In 1846 Von Ebelman's synthesized tetraethyl orthosilicate (Si(OC2H5)4).[28][27]
Silicon in its more common crystalline form was not prepared until 31 years later, by Deville.[29][30] By electrolyzing a mixture of sodium chloride and aluminium chloride containing approximately 10% silicon, he was able to obtain a slightly impure allotrope of silicon in 1854.[31] Later, more cost-effective methods have been developed to isolate several allotrope forms, the most recent being silicene in 2010.[32][33] Meanwhile, research on the chemistry of silicon continued; Friedrich Wöhler discovered the first volatile hydrides of silicon, synthesising trichlorosilane in 1857 and silane itself in 1858, but a detailed investigation of the silanes was only carried out in the early 20th century by Alfred Stock, despite early speculation on the matter dating as far back as the beginnings of synthetic organic chemistry in the 1830s.[34][35] Similarly, the first organosilicon compound, tetraethylsilane, was synthesised by Charles Friedel and James Crafts in 1863, but detailed characterisation of organosilicon chemistry was only done in the early 20th century by Frederic Kipping.[19]
Starting in the 1920s, the work of William Lawrence Bragg on X-ray crystallography elucidated the compositions of the silicates, which had previously been known from analytical chemistry but had not yet been understood, together with Linus Pauling's development of crystal chemistry and Victor Goldschmidt's development of geochemistry. The middle of the 20th century saw the development of the chemistry and industrial use of siloxanes and the growing use of silicone polymers, elastomers, and resins. In the late 20th century, the complexity of the crystal chemistry of silicides was mapped, along with the solid-state physics of doped semiconductors.[19]
Silicon semiconductors
[edit]The first semiconductor devices did not use silicon, but used galena, including German physicist Ferdinand Braun's crystal detector in 1874 and Indian physicist Jagadish Chandra Bose's radio crystal detector in 1901.[36][37] The first silicon semiconductor device was a silicon radio crystal detector, developed by American engineer Greenleaf Whittier Pickard in 1906.[37]
In 1940, Russell Ohl discovered the p–n junction and photovoltaic effects in silicon. In 1941, techniques for producing high-purity germanium and silicon crystals were developed for radar microwave detector crystals during World War II.[36] In 1947, physicist William Shockley theorized a field-effect amplifier made from germanium and silicon, but he failed to build a working device, before eventually working with germanium instead. The first working transistor was a point-contact transistor built by John Bardeen and Walter Brattain later that year while working under Shockley.[38] In 1954, physical chemist Morris Tanenbaum fabricated the first silicon junction transistor at Bell Labs.[39] In 1955, Carl Frosch and Lincoln Derick at Bell Labs accidentally discovered that silicon dioxide (SiO
2) could be grown on silicon.[40][41] By 1957 Frosch and Derick published their work on the first manufactured SiO
2 semiconductor oxide transistor: the first planar transistors, in which drain and source were adjacent at the same surface.[42]
Silicon Age
[edit]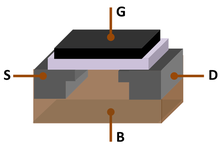
The "Silicon Age" refers to the late 20th century to early 21st century.[44][45][46] This is due to silicon being the dominant material of the Silicon Age (also known as the Digital Age or Information Age), similar to how the Stone Age, Bronze Age and Iron Age were defined by the dominant materials during their respective ages of civilization.[44]
Because silicon is an important element in high-technology semiconductor devices, many places in the world bear its name. For example, the Santa Clara Valley in California acquired the nickname Silicon Valley, as the element is the base material in the semiconductor industry there. Since then, many other places have been similarly dubbed, including Silicon Wadi in Israel; Silicon Forest in Oregon; Silicon Hills in Austin, Texas; Silicon Slopes in Salt Lake City, Utah; Silicon Saxony in Germany; Silicon Valley in India; Silicon Border in Mexicali, Mexico; Silicon Fen in Cambridge, England; Silicon Roundabout in London; Silicon Glen in Scotland; Silicon Gorge in Bristol, England; Silicon Alley in New York City; and Silicon Beach in Los Angeles.[47]
Characteristics
[edit]Physical and atomic
[edit]
A silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. Like the other members of its group, the lighter carbon and the heavier germanium, tin, and lead, it has the same number of valence electrons as valence orbitals: hence, it can complete its octet and obtain the stable noble gas configuration of argon by forming sp3 hybrid orbitals, forming tetrahedral SiX
4 derivatives where the central silicon atom shares an electron pair with each of the four atoms it is bonded to.[49] The first four ionisation energies of silicon are 786.3, 1576.5, 3228.3, and 4354.4 kJ/mol respectively; these figures are high enough to preclude the possibility of simple cationic chemistry for the element. Following periodic trends, its single-bond covalent radius of 117.6 pm is intermediate between those of carbon (77.2 pm) and germanium (122.3 pm). The hexacoordinate ionic radius of silicon may be considered to be 40 pm, although this must be taken as a purely notional figure given the lack of a simple Si4+
cation in reality.[50]
Electrical
[edit]At standard temperature and pressure, silicon is a shiny semiconductor with a bluish-grey metallic lustre; as typical for semiconductors, its resistivity drops as temperature rises. This arises because silicon has a small energy gap (band gap) between its highest occupied energy levels (the valence band) and the lowest unoccupied ones (the conduction band). The Fermi level is about halfway between the valence and conduction bands and is the energy at which a state is as likely to be occupied by an electron as not. Hence pure silicon is effectively an insulator at room temperature. However, doping silicon with a pnictogen such as phosphorus, arsenic, or antimony introduces one extra electron per dopant and these may then be excited into the conduction band either thermally or photolytically, creating an n-type semiconductor. Similarly, doping silicon with a group 13 element such as boron, aluminium, or gallium results in the introduction of acceptor levels that trap electrons that may be excited from the filled valence band, creating a p-type semiconductor.[51] Joining n-type silicon to p-type silicon creates a p–n junction with a common Fermi level; electrons flow from n to p, while holes flow from p to n, creating a voltage drop. This p–n junction thus acts as a diode that can rectify alternating current that allows current to pass more easily one way than the other. A transistor is an n–p–n junction, with a thin layer of weakly p-type silicon between two n-type regions. Biasing the emitter through a small forward voltage and the collector through a large reverse voltage allows the transistor to act as a triode amplifier.[51]
Crystal structure
[edit]Silicon crystallises in a giant covalent structure at standard conditions, specifically in a diamond cubic crystal lattice (space group 227). It thus has a high melting point of 1414 °C, as a lot of energy is required to break the strong covalent bonds and melt the solid. Upon melting silicon contracts as the long-range tetrahedral network of bonds breaks up and the voids in that network are filled in, similar to water ice when hydrogen bonds are broken upon melting. It does not have any thermodynamically stable allotropes at standard pressure, but several other crystal structures are known at higher pressures. The general trend is one of increasing coordination number with pressure, culminating in a hexagonal close-packed allotrope at about 40 gigapascals known as Si–VII (the standard modification being Si–I). An allotrope called BC8 (or bc8), having a body-centred cubic lattice with eight atoms per primitive unit cell (space group 206), can be created at high pressure and remains metastable at low pressure. Its properties have been studied in detail.[52]
Silicon boils at 3265 °C: this, while high, is still lower than the temperature at which its lighter congener carbon sublimes (3642 °C) and silicon similarly has a lower heat of vaporisation than carbon, consistent with the fact that the Si–Si bond is weaker than the C–C bond.[51]
It is also possible to construct silicene layers analogous to graphene.[32][33]
Isotopes
[edit]Naturally occurring silicon is composed of three stable isotopes, 28Si (92.23%), 29Si (4.67%), and 30Si (3.10%).[13] Out of these, only 29Si is of use in NMR and EPR spectroscopy,[53] as it is the only one with a nuclear spin (I =1/2).[34] All three are produced in Type Ia supernovae[54][55] through the oxygen-burning process, with 28Si being made as part of the alpha process and hence the most abundant. The fusion of 28Si with alpha particles by photodisintegration rearrangement in stars is known as the silicon-burning process; it is the last stage of stellar nucleosynthesis before the rapid collapse and violent explosion of the star in question in a type II supernova.[56]
Twenty-two radioisotopes have been characterized, the two stablest being 32Si with a half-life of about 150 years, and 31Si with a half-life of 2.62 hours.[13] All the remaining radioactive isotopes have half-lives that are less than seven seconds, and the majority of these have half-lives that are less than one-tenth of a second.[13] Silicon has one known nuclear isomer, 34mSi, with a half-life less than 210 nanoseconds.[13] 32Si undergoes low-energy beta decay to 32P and then stable 32S. 31Si may be produced by the neutron activation of natural silicon and is thus useful for quantitative analysis; it can be easily detected by its characteristic beta decay to stable 31P, in which the emitted electron carries up to 1.48 MeV of energy.[34]
The known isotopes of silicon range in mass number from 22 to 46.[13][57] The most common decay mode of the isotopes with mass numbers lower than the three stable isotopes is inverse beta decay, primarily forming aluminium isotopes (13 protons) as decay products.[13] The most common decay mode for the heavier unstable isotopes is beta decay, primarily forming phosphorus isotopes (15 protons) as decay products.[13]
Silicon can enter the oceans through groundwater and riverine transport. Large fluxes of groundwater input have an isotopic composition which is distinct from riverine silicon inputs. Isotopic variations in groundwater and riverine transports contribute to variations in oceanic 30Si values. Currently, there are substantial differences in the isotopic values of deep water in the world's ocean basins. Between the Atlantic and Pacific oceans, there is a deep water 30Si gradient of greater than 0.3 parts per thousand. 30Si is most commonly associated with productivity in the oceans.[58]
Chemistry and compounds
[edit]| X = | C | Si | H | F | Cl | Br | I | O– | N< |
|---|---|---|---|---|---|---|---|---|---|
| C–X | 368 | 360 | 435 | 453 | 351 | 293 | 216 | ~360 | ~305 |
| Si–X | 360 | 340 | 393 | 565 | 381 | 310 | 234 | 452 | 322 |
Crystalline bulk silicon is rather inert, but becomes more reactive at high temperatures. Like its neighbour aluminium, silicon forms a thin, continuous surface layer of silicon dioxide (SiO
2) that protects the metal from oxidation. Thus silicon does not measurably react with the air below 900 °C, but formation of the vitreous dioxide rapidly increases between 950 °C and 1160 °C and when 1400 °C is reached, atmospheric nitrogen also reacts to give the nitrides SiN and Si
3N
4. Silicon reacts with gaseous sulfur at 600 °C and gaseous phosphorus at 1000 °C. This oxide layer nevertheless does not prevent reaction with the halogens; fluorine attacks silicon vigorously at room temperature, chlorine does so at about 300 °C, and bromine and iodine at about 500 °C. Silicon does not react with most aqueous acids, but is oxidised and complexed by hydrofluoric acid mixtures containing either chlorine or nitric acid to form hexafluorosilicates. It readily dissolves in hot aqueous alkali to form silicates.[59] At high temperatures, silicon also reacts with alkyl halides; this reaction may be catalysed by copper to directly synthesise organosilicon chlorides as precursors to silicone polymers. Upon melting, silicon becomes extremely reactive, alloying with most metals to form silicides, and reducing most metal oxides because the heat of formation of silicon dioxide is so large. In fact, molten silicon reacts virtually with every known kind of crucible material (except its own oxide, SiO
2).[60]: 13 This happens due to silicon's high binding forces for the light elements and to its high dissolving power for most elements.[60]: 13 As a result, containers for liquid silicon must be made of refractory, unreactive materials such as zirconium dioxide or group 4, 5, and 6 borides.[51][61]
Tetrahedral coordination is a major structural motif in silicon chemistry just as it is for carbon chemistry. However, the 3p subshell is rather more diffuse than the 2p subshell and does not hybridise so well with the 3s subshell. As a result, the chemistry of silicon and its heavier congeners shows significant differences from that of carbon,[62] and thus octahedral coordination is also significant.[51] For example, the electronegativity of silicon (1.90) is much less than that of carbon (2.55), because the valence electrons of silicon are further from the nucleus than those of carbon and hence experience smaller electrostatic forces of attraction from the nucleus. The poor overlap of 3p orbitals also results in a much lower tendency toward catenation (formation of Si–Si bonds) for silicon than for carbon, due to the concomitant weakening of the Si–Si bond compared to the C–C bond:[63] the average Si–Si bond energy is approximately 226 kJ/mol, compared to a value of 356 kJ/mol for the C–C bond.[64] This results in multiply bonded silicon compounds generally being much less stable than their carbon counterparts, an example of the double bond rule. On the other hand, the presence of radial nodes in the 3p orbitals of silicon suggests the possibility of hypervalence, as seen in five and six-coordinate derivatives of silicon such as SiX−
5 and SiF2−
6.[65][63] Lastly, because of the increasing energy gap between the valence s and p orbitals as the group is descended, the divalent state grows in importance from carbon to lead, so that a few unstable divalent compounds are known for silicon; this lowering of the main oxidation state, in tandem with increasing atomic radii, results in an increase of metallic character down the group. Silicon already shows some incipient metallic behavior, particularly in the behavior of its oxide compounds and its reaction with acids as well as bases (though this takes some effort), and is hence often referred to as a metalloid rather than a nonmetal.[63] Germanium shows more, and tin is generally considered a metal.[19]
Silicon shows clear differences from carbon. For example, organic chemistry has very few analogies with silicon chemistry, while silicate minerals have a structural complexity unseen in oxocarbons.[66] Silicon tends to resemble germanium far more than it does carbon, and this resemblance is enhanced by the d-block contraction, resulting in the size of the germanium atom being much closer to that of the silicon atom than periodic trends would predict.[67] Nevertheless, there are still some differences because of the growing importance of the divalent state in germanium compared to silicon. Additionally, the lower Ge–O bond strength compared to the Si–O bond strength results in the absence of "germanone" polymers that would be analogous to silicone polymers.[64]
Occurrence
[edit]
Silicon is the eighth most abundant element in the universe, coming after hydrogen, helium, carbon, nitrogen, oxygen, iron, and neon. These abundances are not replicated well on Earth due to substantial separation of the elements taking place during the formation of the Solar System. Silicon makes up 27.2% of the Earth's crust by weight, second only to oxygen at 45.5%, with which it always is associated in nature. Further fractionation took place in the formation of the Earth by planetary differentiation: Earth's core, which makes up 31.5% of the mass of the Earth, has approximate composition Fe
25Ni
2Co
0.1S
3; the mantle makes up 68.1% of the Earth's mass and is composed mostly of denser oxides and silicates, an example being olivine, (Mg,Fe)
2SiO
4; while the lighter siliceous minerals such as aluminosilicates rise to the surface and form the crust, making up 0.4% of the Earth's mass.[68][69]
The crystallisation of igneous rocks from magma depends on a number of factors; among them are the chemical composition of the magma, the cooling rate, and some properties of the individual minerals to be formed, such as lattice energy, melting point, and complexity of their crystal structure. As magma is cooled, olivine appears first, followed by pyroxene, amphibole, biotite mica, orthoclase feldspar, muscovite mica, quartz, zeolites, and finally, hydrothermal minerals. This sequence shows a trend toward increasingly complex silicate units with cooling, and the introduction of hydroxide and fluoride anions in addition to oxides. Many metals may substitute for silicon. After these igneous rocks undergo weathering, transport, and deposition, sedimentary rocks like clay, shale, and sandstone are formed. Metamorphism also may occur at high temperatures and pressures, creating an even vaster variety of minerals.[68]
There are four sources for silicon fluxes into the ocean: chemical weathering of continental rocks, river transport, dissolution of continental terrigenous silicates, and the reaction between submarine basalts and hydrothermal fluid which release dissolved silicon. All four of these fluxes are interconnected in the ocean's biogeochemical cycle as they all were initially formed from the weathering of Earth's crust.[70]
Approximately 300–900 megatonnes of Aeolian dust is deposited into the world's oceans each year. Of that value, 80–240 megatonnes are in the form of particulate silicon. The total amount of particulate silicon deposition into the ocean is still less than the amount of silicon influx into the ocean via riverine transportation.[71] Aeolian inputs of particulate lithogenic silicon into the North Atlantic and Western North Pacific oceans are the result of dust settling on the oceans from the Sahara and Gobi Desert, respectively.[70] Riverine transports are the major source of silicon influx into the ocean in coastal regions, while silicon deposition in the open ocean is greatly influenced by the settling of Aeolian dust.[71]
Production
[edit]Silicon of 96–99% purity is made by carbothermically reducing quartzite or sand with highly pure coke. The reduction is carried out in an electric arc furnace, with an excess of SiO
2 used to stop silicon carbide (SiC) from accumulating:[34]
- SiO
2 + 2 C → Si + 2 CO - 2 SiC + SiO
2 → 3 Si + 2 CO

This reaction, known as carbothermal reduction of silicon dioxide, usually is conducted in the presence of scrap iron with low amounts of phosphorus and sulfur, producing ferrosilicon.[34] Ferrosilicon, an iron-silicon alloy that contains varying ratios of elemental silicon and iron, accounts for about 80% of the world's production of elemental silicon, with China, the leading supplier of elemental silicon, providing 4.6 million tonnes (or 2/3rds of world output) of silicon, most of it in the form of ferrosilicon. It is followed by Russia (610,000 t), Norway (330,000 t), Brazil (240,000 t), and the United States (170,000 t).[72] Ferrosilicon is primarily used by the iron and steel industry (see below) with primary use as alloying addition in iron or steel and for de-oxidation of steel in integrated steel plants.[34]
Another reaction, sometimes used, is aluminothermal reduction of silicon dioxide, as follows:[73]
- 3 SiO
2 + 4 Al → 3 Si + 2 Al
2O
3
Leaching powdered 96–97% pure silicon with water results in ~98.5% pure silicon, which is used in the chemical industry. However, even greater purity is needed for semiconductor applications, and this is produced from the reduction of tetrachlorosilane (silicon tetrachloride) or trichlorosilane. The former is made by chlorinating scrap silicon and the latter is a byproduct of silicone production. These compounds are volatile and hence can be purified by repeated fractional distillation, followed by reduction to elemental silicon with very pure zinc metal as the reducing agent. The spongy pieces of silicon thus produced are melted and then grown to form cylindrical single crystals, before being purified by zone refining. Other routes use the thermal decomposition of silane or tetraiodosilane (SiI
4). Another process used is the reduction of sodium hexafluorosilicate, a common waste product of the phosphate fertilizer industry, by metallic sodium: this is highly exothermic and hence requires no outside energy source. Hyperfine silicon is made at a higher purity than almost any other material: transistor production requires impurity levels in silicon crystals less than 1 part per 1010, and in special cases impurity levels below 1 part per 1012 are needed and attained.[34]
Silicon nanostructures can directly be produced from silica sand using conventional metalothermic processes, or the combustion synthesis approach. Such nanostructured silicon materials can be used in various functional applications including the anode of lithium-ion batteries (LIBs), other ion batteries, future computing devices like memristors or photocatalytic applications.[74]
Applications
[edit]Compounds
[edit]Most silicon is used industrially without being purified, often with comparatively little processing from its natural form. More than 90% of the Earth's crust is composed of silicate minerals, which are compounds of silicon and oxygen, often with metallic ions when negatively charged silicate anions require cations to balance the charge. Many of these have direct commercial uses, such as clays, silica sand, and most kinds of building stone. Thus, the vast majority of uses for silicon are as structural compounds, either as the silicate minerals or silica (crude silicon dioxide). Silicates are used in making Portland cement (made mostly of calcium silicates) which is used in building mortar and modern stucco, but more importantly, combined with silica sand, and gravel (usually containing silicate minerals such as granite), to make the concrete that is the basis of most of the very largest industrial building projects of the modern world.[75]
Silica is used to make fire brick, a type of ceramic. Silicate minerals are also in whiteware ceramics, an important class of products usually containing various types of fired clay minerals (natural aluminium phyllosilicates). An example is porcelain, which is based on the silicate mineral kaolinite. Traditional glass (silica-based soda–lime glass) also functions in many of the same ways, and also is used for windows and containers. In addition, specialty silica based glass fibers are used for optical fiber, as well as to produce fiberglass for structural support and glass wool for thermal insulation.
Silicones often are used in waterproofing treatments, molding compounds, mold-release agents, mechanical seals, high temperature greases and waxes, and caulking compounds. Silicone is also sometimes used in breast implants, contact lenses, explosives and pyrotechnics.[76] Silly Putty was originally made by adding boric acid to silicone oil.[77] Other silicon compounds function as high-technology abrasives and new high-strength ceramics based upon silicon carbide. Silicon is a component of some superalloys.
Alloys
[edit]Elemental silicon is added to molten cast iron as ferrosilicon or silicocalcium alloys to improve performance in casting thin sections and to prevent the formation of cementite where exposed to outside air. The presence of elemental silicon in molten iron acts as a sink for oxygen, so that the steel carbon content, which must be kept within narrow limits for each type of steel, can be more closely controlled. Ferrosilicon production and use is a monitor of the steel industry, and although this form of elemental silicon is grossly impure, it accounts for 80% of the world's use of free silicon. Silicon is an important constituent of transformer steel, modifying its resistivity and ferromagnetic properties.
The properties of silicon may be used to modify alloys with metals other than iron. "Metallurgical grade" silicon is silicon of 95–99% purity. About 55% of the world consumption of metallurgical purity silicon goes for production of aluminium-silicon alloys (silumin alloys) for aluminium part casts, mainly for use in the automotive industry. Silicon's importance in aluminium casting is that a significantly high amount (12%) of silicon in aluminium forms a eutectic mixture which solidifies with very little thermal contraction. This greatly reduces tearing and cracks formed from stress as casting alloys cool to solidity. Silicon also significantly improves the hardness and thus wear-resistance of aluminium.[78][79]
Electronics
[edit]
Most elemental silicon produced remains as a ferrosilicon alloy, and only approximately 20% is refined to metallurgical grade purity (a total of 1.3–1.5 million metric tons/year). An estimated 15% of the world production of metallurgical grade silicon is further refined to semiconductor purity.[79] This typically is the "nine-9" or 99.9999999% purity,[80] nearly defect-free single crystalline material.[81]
Monocrystalline silicon of such purity is usually produced by the Czochralski process, and is used to produce silicon wafers used in the semiconductor industry, in electronics, and in some high-cost and high-efficiency photovoltaic applications.[82] Pure silicon is an intrinsic semiconductor, which means that unlike metals, it conducts electron holes and electrons released from atoms by heat; silicon's electrical conductivity increases with higher temperatures. Pure silicon has too low a conductivity (i.e., too high a resistivity) to be used as a circuit element in electronics. In practice, pure silicon is doped with small concentrations of certain other elements, which greatly increase its conductivity and adjust its electrical response by controlling the number and charge (positive or negative) of activated carriers. Such control is necessary for transistors, solar cells, semiconductor detectors, and other semiconductor devices used in the computer industry and other technical applications.[83] In silicon photonics, silicon may be used as a continuous wave Raman laser medium to produce coherent light.[84]
In common integrated circuits, a wafer of monocrystalline silicon serves as a mechanical support for the circuits, which are created by doping and insulated from each other by thin layers of silicon oxide, an insulator that is easily produced on Si surfaces by processes of thermal oxidation or local oxidation (LOCOS), which involve exposing the element to oxygen under the proper conditions that can be predicted by the Deal–Grove model. Silicon has become the most popular material for both high power semiconductors and integrated circuits because it can withstand the highest temperatures and greatest electrical activity without suffering avalanche breakdown (an electron avalanche is created when heat produces free electrons and holes, which in turn pass more current, which produces more heat). In addition, the insulating oxide of silicon is not soluble in water, which gives it an advantage over germanium (an element with similar properties which can also be used in semiconductor devices) in certain fabrication techniques.[85]
Monocrystalline silicon is expensive to produce, and is usually justified only in production of integrated circuits, where tiny crystal imperfections can interfere with tiny circuit paths. For other uses, other types of pure silicon may be employed. These include hydrogenated amorphous silicon and upgraded metallurgical-grade silicon (UMG-Si) used in the production of low-cost, large-area electronics in applications such as liquid crystal displays and of large-area, low-cost, thin-film solar cells. Such semiconductor grades of silicon are either slightly less pure or polycrystalline rather than monocrystalline, and are produced in comparable quantities as the monocrystalline silicon: 75,000 to 150,000 metric tons per year. The market for the lesser grade is growing more quickly than for monocrystalline silicon. By 2013, polycrystalline silicon production, used mostly in solar cells, was projected to reach 200,000 metric tons per year, while monocrystalline semiconductor grade silicon was expected to remain less than 50,000 tons per year.[79]
Quantum dots
[edit]Silicon quantum dots are created through the thermal processing of hydrogen silsesquioxane into nanocrystals ranging from a few nanometers to a few microns, displaying size dependent luminescent properties.[86][87] The nanocrystals display large Stokes shifts converting photons in the ultraviolet range to photons in the visible or infrared, depending on the particle size, allowing for applications in quantum dot displays and luminescent solar concentrators due to their limited self absorption. A benefit of using silicon based quantum dots over cadmium or indium is the non-toxic, metal-free nature of silicon.[88][89] Another application of silicon quantum dots is for sensing of hazardous materials. The sensors take advantage of the luminescent properties of the quantum dots through quenching of the photoluminescence in the presence of the hazardous substance.[90] There are many methods used for hazardous chemical sensing with a few being electron transfer, fluorescence resonance energy transfer, and photocurrent generation.[91] Electron transfer quenching occurs when the lowest unoccupied molecular orbital (LUMO) is slightly lower in energy than the conduction band of the quantum dot, allowing for the transfer of electrons between the two, preventing recombination of the holes and electrons within the nanocrystals. The effect can also be achieved in reverse with a donor molecule having its highest occupied molecular orbital (HOMO) slightly higher than a valence band edge of the quantum dot, allowing electrons to transfer between them, filling the holes and preventing recombination. Fluorescence resonance energy transfer occurs when a complex forms between the quantum dot and a quencher molecule. The complex will continue to absorb light but when the energy is converted to the ground state it does not release a photon, quenching the material. The third method uses different approach by measuring the photocurrent emitted by the quantum dots instead of monitoring the photoluminescent display. If the concentration of the desired chemical increases then the photocurrent given off by the nanocrystals will change in response.[92]
Thermal energy storage
[edit]Biological role
[edit]
Although silicon is readily available in the form of silicates, very few organisms use it directly. Diatoms, radiolaria, and siliceous sponges use biogenic silica as a structural material for their skeletons. Some plants accumulate silica in their tissues and require silicon for their growth, for example rice. Silicon may be taken up by plants as orthosilicic acid (also known as monosilicic acid) and transported through the xylem, where it forms amorphous complexes with components of the cell wall. This has been shown to improve cell wall strength and structural integrity in some plants, thereby reducing insect herbivory and pathogenic infections. In certain plants, silicon may also upregulate the production of volatile organic compounds and phytohormones which play a significant role in plant defense mechanisms.[95][96][97] In more advanced plants, the silica phytoliths (opal phytoliths) are rigid microscopic bodies occurring in the cell.[98][99][96]
Several horticultural crops are known to protect themselves against fungal plant pathogens with silica, to such a degree that fungicide application may fail unless accompanied by sufficient silicon nutrition. Silicaceous plant defense molecules activate some phytoalexins, meaning some of them are signalling substances producing acquired immunity. When deprived, some plants will substitute with increased production of other defensive substances.[96]
Life on Earth is largely composed of carbon, but astrobiology considers that extraterrestrial life may have other hypothetical types of biochemistry. Silicon is considered an alternative to carbon, as it can create complex and stable molecules with four covalent bonds, required for a DNA-analog, and it is available in large quantities.[100]
Marine microbial influences
[edit]Diatoms use silicon in the biogenic silica (bSi) form,[101] which is taken up by the silicon transport protein (SIT) to be predominantly used in the cell wall structure as frustules.[102] Silicon enters the ocean in a dissolved form such as silicic acid or silicate.[103] Since diatoms are one of the main users of these forms of silicon, they contribute greatly to the concentration of silicon throughout the ocean. Silicon forms a nutrient-like profile in the ocean due to the diatom productivity in shallow depths.[103] Therefore, concentration of silicon is lower in the shallow ocean and higher in the deep ocean.
Diatom productivity in the upper ocean contributes to the amount of silicon exported to the lower ocean.[104] When diatom cells are lysed in the upper ocean, their nutrients such as iron, zinc, and silicon, are brought to the lower ocean through a process called marine snow. Marine snow involves the downward transfer of particulate organic matter by vertical mixing of dissolved organic matter.[105] It has been suggested that silicon is considered crucial to diatom productivity and as long as there is silicic acid available for diatoms to use, the diatoms can contribute to other important nutrient concentrations in the deep ocean as well.[106]
In coastal zones, diatoms serve as the major phytoplanktonic organisms and greatly contribute to biogenic silica production. In the open ocean, however, diatoms have a reduced role in global annual silica production. Diatoms in North Atlantic and North Pacific subtropical gyres only contribute about 5–7% of global annual marine silica production. The Southern Ocean produces about one-third of global marine biogenic silica.[70] The Southern Ocean is referred to as having a "biogeochemical divide"[107] since only minuscule amounts of silicon are transported out of this region.
Human nutrition
[edit]There is some evidence that silicon is important to human health for their nail, hair, bone, and skin tissues,[108] for example, in studies that demonstrate that premenopausal women with higher dietary silicon intake have higher bone density, and that silicon supplementation can increase bone volume and density in patients with osteoporosis.[109] Silicon is needed for synthesis of elastin and collagen, of which the aorta contains the greatest quantity in the human body,[110] and has been considered an essential element;[111] nevertheless, it is difficult to prove its essentiality, because silicon is very common, and hence, deficiency symptoms are difficult to reproduce.[112][113]
Silicon is currently under consideration for elevation to the status of a "plant beneficial substance by the Association of American Plant Food Control Officials (AAPFCO)."[114][115]
Safety
[edit]People may be exposed to elemental silicon in the workplace by breathing it in, swallowing it, or having contact with the skin or eye. In the latter two cases, silicon poses a slight hazard as an irritant. It is hazardous if inhaled.[116] The Occupational Safety and Health Administration (OSHA) has set the legal limit for silicon exposure in the workplace as 15 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an eight-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set a recommended exposure limit (REL) of 10 mg/m3 total exposure and 5 mg/m3 respiratory exposure over an eight-hour workday.[117] Inhalation of crystalline silica dust may lead to silicosis, an occupational lung disease marked by inflammation and scarring in the form of nodular lesions in the upper lobes of the lungs.[118]
See also
[edit]Notes
[edit]- ^ Although carbon remains solid at higher temperatures than silicon, it sublimes at atmospheric pressure instead of melting and boiling, so it has no melting point and boiling point.
References
[edit]- ^ "Standard Atomic Weights: Silicon". CIAAW. 2009.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ a b c d e f Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 28. ISBN 978-0-08-037941-8.
- ^ "New Type of Zero-Valent Tin Compound". Chemistry Europe. 27 August 2016.
- ^ Ram, R. S.; et al. (1998). "Fourier Transform Emission Spectroscopy of the A2D–X2P Transition of SiH and SiD" (PDF). J. Mol. Spectr. 190 (2): 341–352. doi:10.1006/jmsp.1998.7582. PMID 9668026.
- ^ Eranna, Golla (2014). Crystal Growth and Evaluation of Silicon for VLSI and ULSI. CRC Press. p. 7. ISBN 978-1-4822-3281-3.
- ^ Magnetic susceptibility of the elements and inorganic compounds, in Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ a b c d Hopcroft, Matthew A.; Nix, William D.; Kenny, Thomas W. (2010). "What is the Young's Modulus of Silicon?". Journal of Microelectromechanical Systems. 19 (2): 229. doi:10.1109/JMEMS.2009.2039697.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements: XII. Other elements isolated with the aid of potassium and sodium: beryllium, boron, silicon, and aluminum". Journal of Chemical Education. 9 (8): 1386–1412. Bibcode:1932JChEd...9.1386W. doi:10.1021/ed009p1386.
- ^ Voronkov, M. G. (2007). "Silicon era". Russian Journal of Applied Chemistry. 80 (12): 2190. doi:10.1134/S1070427207120397.
- ^ a b c d e f g h Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Nabil, Marwa; Elnouby, Mohamed; Al-Askar, Abdulaziz A.; Kowalczewski, Przemysław Łukasz; Abdelkhalek, Ahmed; Behiry, Said I. (2024). "Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity". Open Chemistry. 22 (1). doi:10.1515/chem-2023-0169.
- ^ Kamal 2022
- ^ Cutter, Elizabeth G. (1978). Plant Anatomy. Part 1 Cells and Tissues (2nd ed.). London: Edward Arnold. ISBN 978-0-7131-2639-6.
- ^ "Silicon". Encyclopedia Britannica. Retrieved 22 August 2019.
- ^ In his table of the elements, Lavoisier listed five "salifiable earths", i.e., ores that could be made to react with acids to produce salts (salis = salt, in Latin): chaux (calcium oxide), magnésie (magnesia, magnesium oxide), baryte (barium sulfate), alumine (alumina, aluminium oxide), and silice (silica, silicon dioxide). About these "elements", Lavoisier speculates: "We are probably only acquainted as yet with a part of the metallic substances existing in nature, as all those which have a stronger affinity to oxygen than carbon possesses, are incapable, hitherto, of being reduced to a metallic state, and consequently, being only presented to our observation under the form of oxyds, are confounded with earths. It is extremely probable that barytes, which we have just now arranged with earths, is in this situation; for in many experiments it exhibits properties nearly approaching to those of metallic bodies. It is even possible that all the substances we call earths may be only metallic oxyds, irreducible by any hitherto known process." – from Lavoisier (1799). Elements of Chemistry. Translated by Robert Kerr (4 ed.). Edinburgh, Scotland: William Creec. p. 218. (The original passage appears in: Lavoisier (1789). Traité Élémentaire de Chimie. Vol. 1. Paris: Cuchet. p. 174.
- ^ a b c d e Greenwood & Earnshaw 1997, p. 328.
- ^ Davy, Humphry (1808). "Electro chemical researches, on the decomposition of the earths; with observations on the metals obtained from the alkaline earths, and on the amalgam procured from ammonia". Philosophical Transactions of the Royal Society of London. 98. W. Bowyer and J. Nichols: 333–370.. On p. 353 Davy coins the name "silicium" : "Had I been so fortunate as to have obtained more certain evidences on this subject, and to have procured the metallic substances I was in search of, I should have proposed for them the names of silicium [silicon], alumium [aluminium], zirconium, and glucium [beryllium]."
- ^ "14 Silicon". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Gay-Lussac, Joseph Louis; Thénard, Louis Jacques baron (1811). Recherches physico-chimiques, faites sur la pile: sur la préparation chimique et les propriétés du potassium et du sodium; sur la décomposition de l'acide boracique; sur les acides fluorique, muriatique et muriatique oxigéné; sur l'action chimique de la lumière; sur l'analyse végétale et animale, etc (in French). Deterville. pp. 313–314; vol. 2, pp. 55–65.
- ^ Thomson, Thomas; Baldwin, Charles; Blackwood, William; Baldwin, Cradock; Bell & Bradfute, bookseller; Hodges & McArthur, bookseller (1817). A system of chemistry : in four volumes. University of Wisconsin - Madison. London : Printed for Baldwin, Craddock, and Joy, Paternoster-Row; William Blackwood, and Bell and Bradfute, Edinburgh; and Hodges and Macarthur, Dublin. p. 252.: "The base of silica has been usually considered as a metal, and called silicium. But as there is not the smallest evidence for its metallic nature, and as it bears a close resemblance to boron and carbon, it is better to class it along with these bodies, and to give it the name of silicon."
- ^ See
- Berzelius announced his discovery of silicon ("silicium") in: Berzelius, J. (presented: 1823; published: 1824) "Undersökning af flusspatssyran och dess märkvärdigaste föreningar" (Investigation of hydrofluoric acid and of its most noteworthy compounds), Kongliga Vetenskaps-Academiens Handlingar [Proceedings of the Royal Science Academy], 12 : 46–98. The isolation of silicon and its characterization are detailed in the section titled "Flussspatssyrad kisseljords sönderdelning med kalium," pp. 46–68.
- The above article was printed in German in: J.J. Berzelius (1824) "II. Untersuchungen über Flussspathsäure und deren merkwürdigsten Verbindungen" (II. Investigations of hydrofluoric acid and its most noteworthy compounds), Annalen der Physik, 77: 169–230. The isolation of silicon is detailed in the section titled: "Zersetzung der flussspaths. Kieselerde durch Kalium" (Decomposition of silicate fluoride by potassium), pp. 204–210.
- The above article was reprinted in French in: Berzelius (1824) "Décomposition du fluate de silice par le potassium" (Decomposition of silica fluoride by potassium), Annales de Chimie et de Physique, 27: 337–359.
- Reprinted in English in: "On the mode of obtaining silicium, and on the characters and properties of that substance". The Philosophical Magazine and Journal: Comprehending Various Branches of Science, the Liberal and Fine Arts, Agriculture, Manufactures, and Commerce. 65. Richard Taylor and Company: 254–267. 1825.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements: XII. Other elements isolated with the aid of potassium and sodium: beryllium, boron, silicon, and aluminum". Journal of Chemical Education. 9 (8): 1386–1412. Bibcode:1932JChEd...9.1386W. doi:10.1021/ed009p1386.
- ^ Voronkov, M.G. (2007). "Silicon era". Russian Journal of Applied Chemistry. 80 (12): 2190. doi:10.1134/S1070427207120397. S2CID 195240638.
- ^ a b Kipping, Frederic Stanley (1937-03-01). "The bakerian lecture organic derivatives of silicon". Proceedings of the Royal Society of London, Series A. 159 (896): 139–148. Bibcode:1937RSPSA.159..139K. doi:10.1098/rspa.1937.0063.
- ^ Muller, Richard (January 1965). "One hundred years of organosilicon chemistry". Journal of Chemical Education. 42 (1): 41. Bibcode:1965JChEd..42...41M. doi:10.1021/ed042p41. ISSN 0021-9584.
- ^ In 1854, Deville was trying to prepare aluminium from aluminium chloride that was heavily contaminated with silicon chloride. Deville used two methods to prepare aluminium: heating aluminium chloride with sodium metal in an inert atmosphere (of hydrogen); and melting aluminium chloride with sodium chloride and then electrolyzing the mixture. In both cases, pure silicon was produced: the silicon dissolved in the molten aluminium, but crystallized upon cooling. Dissolving the crude aluminium in hydrochloric acid revealed flakes of crystallized silicon. See: Henri Sainte-Claire Deville (1854) "Note sur deux procédés de préparation de l'aluminium et sur une nouvelle forme du silicium" (Note on two procedures for the preparation of aluminium and on a new form of silicon), Comptes rendus, 39: 321–326.
Subsequently, Deville obtained crystalline silicon by heating the chloride or fluoride of silicon with sodium metal, isolating the amorphous silicon, then melting the amorphous form with salt and heating the mixture until most of the salt evaporated. See: Sainte-Claire Deville, H. (1855). "Du silicium et du titane (On silicon and titanium)". Comptes rendus. 40: 1034–1036. - ^ "Information on silicon – history, thermodynamic, chemical, physical and electronic properties". Etacude. Retrieved 2021-06-08.
- ^ "Silicon: History". Nautilus.fis.uc.pt. 2011-07-27. Archived from the original on July 27, 2011.
- ^ a b Aufray, B.; Kara, A.; Vizzini, S. B.; Oughaddou, H.; LéAndri, C.; Ealet, B.; Le Lay, G. (2010). "Graphene-like silicon nanoribbons on Ag(110): A possible formation of silicene". Applied Physics Letters. 96 (18): 183102. Bibcode:2010ApPhL..96r3102A. doi:10.1063/1.3419932.
- ^ a b Lalmi, B.; Oughaddou, H.; Enriquez, H.; Kara, A.; Vizzini, S. B.; Ealet, B. N.; Aufray, B. (2010). "Epitaxial growth of a silicene sheet". Applied Physics Letters. 97 (22): 223109. arXiv:1204.0523. Bibcode:2010ApPhL..97v3109L. doi:10.1063/1.3524215. S2CID 118490651.
- ^ a b c d e f g h Greenwood & Earnshaw 1997, p. 330.
- ^ Greenwood & Earnshaw 1997, pp. 337–340
- ^ a b "Timeline". The Silicon Engine. Computer History Museum. Retrieved 22 August 2019.
- ^ a b "1901: Semiconductor Rectifiers Patented as "Cat's Whisker" Detectors". The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ "1947: Invention of the Point-Contact Transistor". The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ "1954: Morris Tanenbaum fabricates the first silicon transistor at Bell Labs". The Silicon Engine. Computer History Museum. Retrieved 23 August 2019.
- ^ US2802760A, Lincoln, Derick & Frosch, Carl J., "Oxidation of semiconductive surfaces for controlled diffusion", issued 1957-08-13
- ^ Bassett, Ross Knox (2007). To the Digital Age: Research Labs, Start-up Companies, and the Rise of MOS Technology. Johns Hopkins University Press. pp. 22–23. ISBN 978-0-8018-8639-3.
- ^ Frosch, C. J.; Derick, L (1957). "Surface Protection and Selective Masking during Diffusion in Silicon". Journal of the Electrochemical Society. 104 (9): 547. doi:10.1149/1.2428650.
- ^ Frosch, C. J.; Derick, L (1957). "Surface Protection and Selective Masking during Diffusion in Silicon". Journal of the Electrochemical Society. 104 (9): 547. doi:10.1149/1.2428650.
- ^ a b Feldman, Leonard C. (2001). "Introduction". Fundamental Aspects of Silicon Oxidation. Springer Science & Business Media. pp. 1–11. ISBN 978-3-540-41682-1.
- ^ Dabrowski, Jarek; Müssig, Hans-Joachim (2000). "1.2. The Silicon Age". Silicon Surfaces and Formation of Interfaces: Basic Science in the Industrial World. World Scientific. pp. 3–13. ISBN 978-981-02-3286-3.
- ^ Siffert, Paul; Krimmel, Eberhard (2013). "Preface". Silicon: Evolution and Future of a Technology. Springer Science & Business Media. ISBN 978-3-662-09897-4.
- ^ Uskali, T.; Nordfors, D. (23 May 2007). "The role of journalism in creating the metaphor of Silicon Valley" (PDF). Innovation Journalism 4 Conference, Stanford University. Archived from the original (PDF) on 2012-09-07. Retrieved 2016-08-08.
- ^ "Silicon and Germanium". hyperphysics.phy-astr.gsu.edu. Retrieved 2021-06-07.
- ^ King 1995, pp. xiii–xviii
- ^ Greenwood & Earnshaw 1997, pp. 372.
- ^ a b c d e Greenwood & Earnshaw 1997, p. 331.
- ^ Vladimir E. Dmitrienko and Viacheslav A. Chizhikov (2020). "An infinite family of bc8-like metastable phases in silicon". Phys. Rev. B. 101 (24): 245203. arXiv:1912.10672. Bibcode:2020PhRvB.101x5203D. doi:10.1103/PhysRevB.101.245203. S2CID 209444444.
- ^ Jerschow, Alexej. "Interactive NMR Frequency Map". New York University. Retrieved 2011-10-20.
- ^ Seitenzahl, Ivo Rolf; Townsley, Dean M. (2017). "Nucleosynthesis in Thermonuclear Supernovae". Handbook of Supernovae. pp. 1955–1978. arXiv:1704.00415. Bibcode:2017hsn..book.1955S. doi:10.1007/978-3-319-21846-5_87. ISBN 978-3-319-21845-8. S2CID 118993185.
- ^ Khokhlov, A. M.; Oran, E. S.; Wheeler, J. C. (April 1997). "Deflagration-to-Detonation Transition in Thermonuclear Supernovae". The Astrophysical Journal. 478 (2): 678–688. arXiv:astro-ph/9612226. Bibcode:1997ApJ...478..678K. doi:10.1086/303815. S2CID 53486905.
- ^ Cameron, A.G.W. (1973). "Abundance of the Elements in the Solar System" (PDF). Space Science Reviews. 15 (1): 121–146. Bibcode:1973SSRv...15..121C. doi:10.1007/BF00172440. S2CID 120201972. Archived from the original (PDF) on 2011-10-21.
- ^ Yoshimoto, Masahiro; Suzuki, Hiroshi; Fukuda, Naoki; Takeda, Hiroyuki; Shimizu, Yohei; Yanagisawa, Yoshiyuki; Sato, Hiromi; Kusaka, Kensuke; Ohtake, Masao; Yoshida, Koichi; Michimasa, Shin’ichiro (2024). "Discovery of Neutron-Rich Silicon Isotopes 45,46Si". Progress of Theoretical and Experimental Physics. 2024 (10). Oxford University Press (OUP). doi:10.1093/ptep/ptae155. ISSN 2050-3911.
- ^ Reynolds, B. C. (June 2009). "Modeling the modern marine δ 30 Si distribution: MODELING THE MODERN MARINE δ 30 Si DISTRIBUTION". Global Biogeochemical Cycles. 23 (2): 1–13. doi:10.1029/2008GB003266. S2CID 128652214.
- ^ Stapf, André; Gondek, Christoph; Kroke, Edwin; Roewer, Gerhard (2019), Yang, Deren (ed.), "Wafer Cleaning, Etching, and Texturization", Handbook of Photovoltaic Silicon, Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 311–358, doi:10.1007/978-3-662-56472-1_17, ISBN 978-3-662-56471-4, S2CID 226945433, retrieved 2021-03-07
- ^ a b Grabmaier, J. (1982). Silicon Chemical Etching. Berlin, Heidelberg: Springer Berlin Heidelberg. ISBN 978-3-642-68765-5. OCLC 840294227.
- ^ Greenwood & Earnshaw 1997, pp. 331–5
- ^ Kaupp, Martin (1 December 2006). "The role of radial nodes of atomic orbitals for chemical bonding and the periodic table" (PDF). Journal of Computational Chemistry. 28 (1): 320–325. doi:10.1002/jcc.20522. PMID 17143872. S2CID 12677737. Retrieved 14 October 2016.
- ^ a b c King 1995, pp. 43–44
- ^ a b Greenwood & Earnshaw 1997, pp. 374.
- ^ Kaupp, Martin (2007). "The role of radial nodes of atomic orbitals for chemical bonding and the periodic table". Journal of Computational Chemistry. 28 (1): 320–325. doi:10.1002/jcc.20522. ISSN 0192-8651. PMID 17143872.
- ^ Greenwood & Earnshaw 1997, pp. 327–328.
- ^ Greenwood & Earnshaw 1997, pp. 359–361.
- ^ a b Greenwood & Earnshaw 1997, p. 329.
- ^ Greenwood & Earnshaw 1997, pp. 329–330
- ^ a b c Tréguer, Paul J.; De La Rocha, Christina L. (3 January 2013). "The World Ocean Silica Cycle". Annual Review of Marine Science. 5 (1): 477–501. doi:10.1146/annurev-marine-121211-172346. PMID 22809182.
- ^ a b Tegen, Ina; Kohfeld, Karen (2006). Atmospheric transport of silicon. Island Press. pp. 81–91. ISBN 1-59726-115-7.
- ^ "Silicon Commodities Report 2011" (PDF). USGS. Retrieved 2011-10-20.
- ^ Zulehner, Neuer & Rau, p. 574
- ^ Kamali, A.R. (2019). "Ultra-fast shock-wave combustion synthesis of nanostructured silicon from sand with excellent Li storage performance". Sustainable Energy & Fuels. 3 (6): 1396–1405. doi:10.1039/C9SE00046A. S2CID 139986478.
- ^ Greenwood & Earnshaw 1997, p. 356.
- ^ Koch, E.C.; Clement, D. (2007). "Special Materials in Pyrotechnics: VI. Silicon – An Old Fuel with New Perspectives". Propellants, Explosives, Pyrotechnics. 32 (3): 205. doi:10.1002/prep.200700021.
- ^ Walsh, Tim (2005). "Silly Putty". Timeless toys: classic toys and the playmakers who created them. Andrews McMeel Publishing. ISBN 978-0-7407-5571-2.
- ^ Apelian, D. (2009). "Aluminum Cast Alloys: Enabling Tools for Improved Performance" (PDF). Wheeling, Illinois: North American Die Casting Association. Archived from the original (PDF) on 2012-01-06.
- ^ a b c Corathers, Lisa A. 2009 Minerals Yearbook. USGS
- ^ "Semi" SemiSource 2006: A supplement to Semiconductor International. December 2005. Reference Section: How to Make a Chip. Adapted from Design News. Reed Electronics Group.
- ^ SemiSource 2006: A supplement to Semiconductor International. December 2005. Reference Section: How to Make a Chip. Adapted from Design News. Reed Electronics Group.
- ^ Zulehner, Neuer & Rau, p. 590
- ^ Zulehner, Neuer & Rau, p. 573
- ^ Dekker, R; Usechak, N; Först, M; Driessen, A (2008). "Ultrafast nonlinear all-optical processes in silicon-on-insulator waveguides" (PDF). Journal of Physics D. 40 (14): R249–R271. Bibcode:2007JPhD...40..249D. doi:10.1088/0022-3727/40/14/r01. S2CID 123008652. Archived from the original (PDF) on 2024-04-16. Retrieved 2024-04-15.
- ^ Semiconductors Without the Quantum Physics Archived 2021-08-13 at the Wayback Machine. Electropaedia
- ^ Clark, Rhett J.; Aghajamali, Maryam; Gonzalez, Christina M.; Hadidi, Lida; Islam, Muhammad Amirul; Javadi, Morteza; Mobarok, Md Hosnay; Purkait, Tapas K.; Robidillo, Christopher Jay T.; Sinelnikov, Regina; Thiessen, Alyxandra N. (2017-01-10). "From Hydrogen Silsesquioxane to Functionalized Silicon Nanocrystals". Chemistry of Materials. 29 (1): 80–89. doi:10.1021/acs.chemmater.6b02667. ISSN 0897-4756.
- ^ Hessel, Colin M.; Henderson, Eric J.; Veinot, Jonathan G. C. (2007). "Hydrogen Silsesquioxane: A Molecular Precursor for Nanocrystalline Si—SiO2 Composites and Freestanding Hydride-Surface-Terminated Silicon Nanoparticles". ChemInform. 38 (10). doi:10.1002/chin.200710014. ISSN 1522-2667.
- ^ Lim, Cheol Hong; Han, Jeong-Hee; Cho, Hae-Won; Kang, Mingu (2014). "Studies on the Toxicity and Distribution of Indium Compounds According to Particle Size in Sprague-Dawley Rats". Toxicological Research. 30 (1): 55–63. Bibcode:2014ToxRe..30...55L. doi:10.5487/TR.2014.30.1.055. ISSN 1976-8257. PMC 4007045. PMID 24795801.
- ^ Zou, Hui; Wang, Tao; Yuan, Junzhao; Sun, Jian; Yuan, Yan; Gu, Jianhong; Liu, Xuezhong; Bian, Jianchun; Liu, Zongping (2020-03-15). "Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes". Toxicology Letters. 321: 32–43. doi:10.1016/j.toxlet.2019.12.019. ISSN 0378-4274. PMID 31862506. S2CID 209435190.
- ^ Nguyen, An; Gonzalez, Christina M; Sinelnikov, Regina; Newman, W; Sun, Sarah; Lockwood, Ross; Veinot, Jonathan G C; Meldrum, Al (2016-02-10). "Detection of nitroaromatics in the solid, solution, and vapor phases using silicon quantum dot sensors". Nanotechnology. 27 (10): 105501. Bibcode:2016Nanot..27j5501N. doi:10.1088/0957-4484/27/10/105501. ISSN 0957-4484. PMID 26863492. S2CID 24292648.
- ^ Gonzalez, Christina M.; Veinot, Jonathan G. C. (2016-06-02). "Silicon nanocrystals for the development of sensing platforms". Journal of Materials Chemistry C. 4 (22): 4836–4846. doi:10.1039/C6TC01159D. ISSN 2050-7534.
- ^ Yue, Zhao; Lisdat, Fred; Parak, Wolfgang J.; Hickey, Stephen G.; Tu, Liping; Sabir, Nadeem; Dorfs, Dirk; Bigall, Nadja C. (2013-04-24). "Quantum-Dot-Based Photoelectrochemical Sensors for Chemical and Biological Detection". ACS Applied Materials & Interfaces. 5 (8): 2800–2814. doi:10.1021/am3028662. ISSN 1944-8244. PMID 23547912.
- ^ "Molten silicon used for thermal energy storage". The Engineer. Archived from the original on 4 November 2016. Retrieved 2 November 2016.
- ^ "Energy-storage system based on silicon from sand". www.powerengineeringint.com. Archived from the original on 4 November 2016. Retrieved 2 November 2016.
- ^ Kim, Sang Gyu; Kim, Ki Woo; Park, Eun Woo; Choi, Doil (2002). "Silicon-Induced Cell Wall Fortification of Rice Leaves: A Possible Cellular Mechanism of Enhanced Host Resistance to Blast". Phytopathology. 92 (10): 1095–103. doi:10.1094/PHYTO.2002.92.10.1095. PMID 18944220.
- ^ a b c Epstein, Emanuel (1999). "SILICON". Annual Review of Plant Physiology and Plant Molecular Biology. 50: 641–664. doi:10.1146/annurev.arplant.50.1.641. PMID 15012222.
- ^ Leroy, Nicolas; de Tombeur, Felix; Walgraffe, Yseult; Cornelis, Jean-Thomas; Verheggen, Francois (23 October 2019). "Silicon and plant natural defenses against insect pests: impact on plant volatile organic compounds and cascade effects on multitrophic interactions". Plants. 8 (444): 444. doi:10.3390/plants8110444. PMC 6918431. PMID 31652861.
- ^ Rahman, Atta-ur- (2008). "Silicon". Studies in Natural Products Chemistry. Vol. 35. Elsevier Science. p. 856. ISBN 978-0-444-53181-0.
- ^ Exley, C. (1998). "Silicon in life:A bioinorganic solution to bioorganic essentiality". Journal of Inorganic Biochemistry. 69 (3): 139–144. doi:10.1016/S0162-0134(97)10010-1.
- ^ Aguilera Mochón, Juan Antonio (2016). La vida no terrestre [The non-terrestrial life] (in Spanish). RBA. pp. 43–45. ISBN 978-84-473-8665-9.
- ^ Bidle, Kay D.; Manganelli, Maura; Azam, Farooq (2002-12-06). "Regulation of Oceanic Silicon and Carbon Preservation by Temperature Control on Bacteria". Science. 298 (5600): 1980–1984. Bibcode:2002Sci...298.1980B. doi:10.1126/science.1076076. ISSN 0036-8075. PMID 12471255. S2CID 216994.
- ^ Durkin, Colleen A.; Koester, Julie A.; Bender, Sara J.; Armbrust, E. Virginia (2016). "The evolution of silicon transporters in diatoms". Journal of Phycology. 52 (5): 716–731. Bibcode:2016JPcgy..52..716D. doi:10.1111/jpy.12441. ISSN 1529-8817. PMC 5129515. PMID 27335204.
- ^ a b Dugdale, R. C.; Wilkerson, F. P. (2001-12-30). "Sources and fates of silicon in the ocean: the role of diatoms in the climate and glacial cycles". Scientia Marina. 65 (S2): 141–152. doi:10.3989/scimar.2001.65s2141. ISSN 1886-8134.
- ^ Baines, Stephen B.; Twining, Benjamin S.; Brzezinski, Mark A.; Krause, Jeffrey W.; Vogt, Stefan; Assael, Dylan; McDaniel, Hannah (December 2012). "Significant silicon accumulation by marine picocyanobacteria". Nature Geoscience. 5 (12): 886–891. Bibcode:2012NatGe...5..886B. doi:10.1038/ngeo1641. ISSN 1752-0908.
- ^ Turner, Jefferson T. (January 2015). "Zooplankton fecal pellets, marine snow, phytodetritus and the ocean's biological pump". Progress in Oceanography. 130: 205–248. Bibcode:2015PrOce.130..205T. doi:10.1016/j.pocean.2014.08.005. ISSN 0079-6611.
- ^ Yool, Andrew; Tyrrell, Toby (2003). "Role of diatoms in regulating the ocean's silicon cycle". Global Biogeochemical Cycles. 17 (4): n/a. Bibcode:2003GBioC..17.1103Y. CiteSeerX 10.1.1.394.3912. doi:10.1029/2002GB002018. ISSN 1944-9224. S2CID 16849373.
- ^ Marinov, I.; Gnanadesikan, A.; Toggweiler, J. R.; Sarmiento, J. L. (June 2006). "The Southern Ocean biogeochemical divide". Nature. 441 (7096): 964–967. Bibcode:2006Natur.441..964M. doi:10.1038/nature04883. PMID 16791191. S2CID 4428683.
- ^ Martin, Keith R. (2013). "Silicon: The Health Benefits of a Metalloid". In Astrid Sigel; Helmut Sigel; Roland K.O. Sigel (eds.). Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. Vol. 13. Springer. pp. 451–473. doi:10.1007/978-94-007-7500-8_14. ISBN 978-94-007-7499-5. PMID 24470100.
- ^ Jugdaohsingh, R. (Mar–Apr 2007). "Silicon and bone health". The Journal of Nutrition, Health and Aging. 11 (2): 99–110. PMC 2658806. PMID 17435952.
- ^ Loeper, J.; Fragny, M. (1978). "The Physiological Role of the Silicon and its AntiAtheromatous Action". Biochemistry of Silicon and Related Problems. pp. 281–296. doi:10.1007/978-1-4613-4018-8_13. ISBN 978-1-4613-4020-1.
- ^ Nielsen, Forrest H. (1984). "Ultratrace Elements in Nutrition". Annual Review of Nutrition. 4: 21–41. doi:10.1146/annurev.nu.04.070184.000321. PMID 6087860.
- ^ Lippard, Stephen J.; Jeremy M. Berg (1994). Principles of Bioinorganic Chemistry. Mill Valley, CA: University Science Books. p. 411. ISBN 978-0-935702-72-9.
- ^ Muhammad Ansar Farooq; Karl-Josef Dietz (2015). "Silicon as Versatile Player in Plant and Human Biology: Overlooked and Poorly Understood Muhammad Ansar Farooq and Karl-J". Front. Plant Sci. 6 (994): 994. doi:10.3389/fpls.2015.00994. PMC 4641902. PMID 26617630.
- ^ "AAPFCO Board of Directors 2006 Mid-Year Meeting" (PDF). Association of American Plant Food Control Officials. Archived from the original (PDF) on 6 January 2012. Retrieved 2011-07-18.
A presentation was made for Excell Minerals to recognize Silicon as a recognized plant nutrient
- ^ Miranda, Stephen R.; Barker, Bruce (August 4, 2009). "Silicon: Summary of Extraction Methods". Harsco Minerals. Archived from the original on November 12, 2012. Retrieved 2011-07-18.
- ^ Science Lab.com. "Material Safety Data Sheet: Silicon MSDS". sciencelab.com. Archived from the original on 23 March 2018. Retrieved 11 March 2018.
- ^ "CDC – NIOSH Pocket Guide to Chemical Hazards – Silicon". www.cdc.gov. Retrieved 2015-11-21.
- ^ Jane A. Plant; Nick Voulvoulis; K. Vala Ragnarsdottir (2012). Pollutants, Human Health and the Environment: A Risk Based Approach. Vol. 26. John Wiley & Sons. p. 273. Bibcode:2011ApGC...26S.238P. doi:10.1016/j.apgeochem.2011.03.113. ISBN 978-0-470-74261-7. Retrieved 24 August 2012.
{{cite book}}:|journal=ignored (help)
Bibliography
[edit]- Clayden, Jonathan; Greeves, Nick; Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford University Press. ISBN 978-0-19-927029-3.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- King, R. Bruce (1995). Inorganic Chemistry of Main Group Elements. Wiley-VCH. ISBN 978-0-471-18602-1.
- Zulehner, Werner; Neuer, Bernd; Rau, Gerhard. "Silicon". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_721. ISBN 978-3527306732.
- Kamal, Kamal Y. (2022). "The Silicon Age: Trends in Semiconductor Devices Industry" (PDF). Journal of Engineering Science and Technology Review. 15 (1): 110–5. doi:10.25103/jestr.151.14. S2CID 249074588.
External links
[edit]- "Silicon Video - The Periodic Table of Videos - University of Nottingham". www.periodicvideos.com. Retrieved 2021-06-08.
- "CDC - NIOSH Pocket Guide to Chemical Hazards - Silicon". www.cdc.gov. Retrieved 2021-06-08.
- "Physical properties of Silicon (Si)". www.ioffe.ru. Retrieved 2021-06-08.
- The Story of Solar-Grade Silicon. Asianometry. 30 November 2022.

