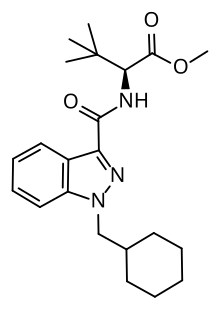
MDMB-CHMINACA

 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H31N3O3 |
| Molar mass | 385.508 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
MDMB-CHMINACA[1] (also known as MDMB(N)-CHM) is an indazole-based synthetic cannabinoid that acts as a potent agonist of the CB1 receptor,[2] and has been sold online as a designer drug.[3][4][5][6][7] It was invented by Pfizer in 2008, and is one of the most potent cannabinoid agonists known, with a binding affinity of 0.0944 nM at CB1, and an EC50 of 0.330 nM.[8] It is closely related to MDMB-FUBINACA, which caused at least 1000 hospitalizations and 40 deaths in Russia as consequence of intoxication.[9]
Legal status
[edit]MDMB-CHMINACA is a Fifth Schedule of the Misuse of Drugs Act (MDA) controlled substance in Singapore as of May 2015.[10]
MDMB-CHMINACA is illegal in Germany, Switzerland as of December 2015.[11]
Sweden's public health agency suggested classifying MDMB-CHMINACA as a hazardous substance, on September 25, 2019.[12]
See also
[edit]- AB-CHMINACA
- ADB-CHMINACA
- ADB-FUBINACA
- MDMB-CHMICA
- MDMB-FUBINACA
- PX-3 (APP-CHMINACA)
References
[edit]- ^ Pulver B, Fischmann S, Gallegos A, Christie R (March 2023). "EMCDDA framework and practical guidance for naming synthetic cannabinoids". Drug Testing and Analysis. 15 (3): 255–276. doi:10.1002/dta.3403. PMID 36346325.
- ^ Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, et al. (September 2016). "Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues". ACS Chemical Neuroscience. 7 (9): 1241–54. doi:10.1021/acschemneuro.6b00137. PMID 27421060.
- ^ "MDMB-CHMINACA". Cayman Chemical. Retrieved 14 July 2015.
- ^ Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Shafran Y, Morzherin Y, et al. (August 2015). "Identification and analytical characteristics of synthetic cannabinoids with an indazole-3-carboxamide structure bearing a N-1-methoxycarbonylalkyl group". Analytical and Bioanalytical Chemistry. 407 (21): 6301–15. doi:10.1007/s00216-015-8612-7. PMID 25893797. S2CID 31838655.
- ^ Savchuk SA (2014). "Detection methods of psychoactive substances and liquid chromotograoghy detection of metabolites" (PDF) (in Russian). Russian Ministry of Health. Retrieved 14 July 2015.
- ^ "Идентификация синтетических каннабимиметиков MDMB-CHMINACA, MDMB-FUBINACA и их метаболитов" [Identification of synthetic cannabimimetics MDMB-CHMINACA, MDMB-FUBINACA and their metabolites] (in Russian). CTS "SCIENCE". Retrieved 14 July 2015.
- ^ Hess C, Murach J, Krueger L, Scharrenbroch L, Unger M, Madea B, et al. (May 2017). "Simultaneous detection of 93 synthetic cannabinoids by liquid chromatography-tandem mass spectrometry and retrospective application to real forensic samples". Drug Testing and Analysis. 9 (5): 721–733. doi:10.1002/dta.2030. PMID 27400642.
- ^ Buchler IP et al, INDAZOLE DERIVATIVES. WO 2009/106980
- ^ "Очередная жертва спайса" [Another victim of spice] (in Russian). Federal Drug Control Service of the Russian Federation. 17 March 2015. Archived from the original on 14 July 2015. Retrieved 13 July 2015.
- ^ "CNB NEWS RELEASE". Central Narcotics Bureau (CNB). 30 April 2015. Archived from the original on 15 July 2015. Retrieved 14 July 2015.
- ^ "Verordnung des EDI über die Verzeichnisse der Betäubungsmittel, psychotropen Stoffe, Vorläuferstoffe und Hilfschemikalien" (in German). Der Bundesrat.
- ^ "Tretton ämnen föreslås klassas som narkotika eller hälsofarlig vara" [Thirteen substances are proposed to be classified as narcotics or dangerous goods] (in Swedish). Folkhälsomyndigheten (The Public Health Authority). 25 September 2019.