PTPRM

Receptor-type tyrosine-protein phosphatase mu is an enzyme that in humans is encoded by the PTPRM gene.[5][6][7]
Function
[edit]The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. Protein tyrosine phosphatases are protein enzymes that remove phosphate moieties from tyrosine residues on other proteins. Tyrosine kinases are enzymes that add phosphates to tyrosine residues, and are the opposing enzymes to PTPs. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. PTPs can be both cytosolic and transmembrane.[8][9]
Structure
[edit]Transmembrane PTPs are known as receptor protein tyrosine phosphatases (RPTPs). RPTPs are single pass transmembrane proteins usually with one or two catalytic domains in their intracellular domain (the part of the protein that is inside the cell) and diverse extracellular structures (the part of the protein that is outside the cell).[10][11]
PTPmu possesses an extracellular region, a single transmembrane region, a 158 amino acid long juxtamembrane domain and two tandem tyrosine phosphatase domains (referred to as D1 and D2) in its intracellular domain, and thus represents an RPTP.[5] Only the membrane proximal phosphatase domain, D1, is catalytically active. The extracellular region contains a meprin-A5 antigen-PTP mu (MAM) domain, an Ig-like domain and four fibronectin type III-like repeats. There are other RPTPs that resemble PTPmu. These proteins are all grouped as type IIb RPTPs, and include PTPkappa (κ), PTPrho (ρ), and PCP-2. The structure of type IIb RPTPs classifies them as members of the immunoglobulin superfamily of cell adhesion molecules, in addition to being tyrosine phosphatases.[10][12] The structure of PTPmu suggests that it can regulate cell adhesion and migration using its extracellular cell adhesion molecule features, while also regulating the level of tyrosine phosphorylation inside of cells using its catalytic tyrosine phosphatase domain. A series of reviews have been written about RPTPs including PTPmu.[10][11][13][14][15][16][17][18][19][20][21] PTPmu is expressed in different organ tissues in the body, including the lung, heart and brain,[22] pancreas,[23] endothelial cells in capillaries and arteries throughout the body,[24][25][26] and in retinal and brain cells.[27][28][29][30][31] PTPmu has been shown to increase the mRNA of the K+ channel Kv1.5 in cardiac myocytes when CHO cells expressing PTPmu are cultured with cardiac myocytes.[32]
Homophilic binding
[edit]PTPmu protein expressed on the surface of cells is able to mediate binding between two cells, which results in the clustering of the cells, known as cell–cell aggregation.[33][34] PTPmu accomplishes this by interacting with another PTPmu molecule on an adjacent cell, known as homophilic binding. The Ig domain of PTPmu is responsible for promoting homophilic binding.[35] The Ig domain is also responsible for localizing PTPmu to the plasma membrane surface of the cell.[36] The ability of closely related molecules like PTPmu and PTPkappa to separate themselves to associate only with their identically matched (homologous) molecules, known as sorting, is attributed to the MAM domain.[37] The MAM, Ig, and the first two FNIII repeats are the minimum extracellular domains required for efficient cell–cell adhesion.[35][36][37][38][39][40][41] Crystallographic studies demonstrated that the MAM and Ig domains are tightly associated into one functional entity.[39] Additional crystal structure analysis by Aricescu and colleagues predicted that the adhesive interface between two PTPμ proteins is between the MAM and Ig domains of one PTPμ protein interacts with the first and second FN III domains of the second PTPμ protein.[40] The type IIb RPTPs mediate adhesion, with the exception of PCP-2.[42]
Tyrosine phosphatase activity
[edit]There are a number of ways that RPTP catalytic activity can be regulated (for reviews, see [11][14][17][43]). Dimerization of identical RPTP proteins at the cell surface leaves the PTP domains either in an open active conformation, as in the case of PTPmu[44] and LAR,[45] or in an inhibited conformation that leaves the catalytic domain inaccessible, in the case of CD45,[46] PTPalpha,[47] and PTPzeta/beta.[48] The binding of different parts of the protein with itself (ex. by folding to interact with itself), known as intramolecular interactions, can affect the activity of RPTPs. The cytoplasmic domains of different RPTPs can interact[49][50] to yield heterodimers of RPTP proteins, which then influence catalytic activity (for example, see [51]).
The regulation of PTPmu catalytic activity is complex. Like most RPTPs, the membrane proximal (or D1) phosphatase domain of PTPmu is catalytically active.[52] At high cell density, when PTPmu molecules bind to one another homophilically, phosphotyrosine levels are decreased.[53] This suggests that PTPmu may be catalytically active at high cell density. Substrates of PTPmu (proteins that are dephosphorylated by PTPmu), such as p120catenin, tend to be dephosphorylated at high cell density,[54] supporting the hypothesis that PTPmu is catalytically active when bound homophilically. PTPmu is constitutively dimerized due to its extracellular domain.[55]
Crystal structure analysis of the D1 of PTPmu demonstrated that PTPmu dimers are in an open active conformation.[44] Even though PTPmu dimers may be active, an additional study suggests that the extracellular domain of PTPmu reduces phosphatase activity. In this study, it was shown that the cytoplasmic domain of PTPmu (a PTPmu molecule lacking the extracellular domain) has greater phosphatase activity than the full-length protein in an enzymatic phosphatase assay.[56]
PTPmu has a long juxtamembrane domain, which likely influences catalytic activity. The juxtamembrane domain of PTPmu can bind to either the D1 and/or D2 of PTPmu, but only within the same PTPmu monomer.[57] Removal of the juxtamembrane domain from PTPmu has been suggested to reduce PTPmu phosphatase activity.[52] The D2 domain of PTPmu also regulates its activity. Although originally demonstrated to positively regulate phosphatase activity,[52] the D2 domain has been shown to negatively affect PTPmu catalytic activity.[58] A wedge-shaped motif located by D1 also regulates catalytic activity.[59] Use of a peptide with the same sequence as the wedge motif inhibits PTPmu mediated functions.[59][60][61][62]
Certain stimuli may also influence PTP activity. For example, alteration of cell oxidation induces conformational changes in the cytoplasmic domain of PTPmu, which may affect its tyrosine phosphatase activity or binding of extracellular ligands.[55]
Cadherin-dependent adhesion
[edit]Classical cadherins are important proteins for cells to bind in the body (‘’in vivo’’) where they commonly stabilize cell–cell junctions known as adherens junctions. Cadherins stabilize adherens junctions through the interaction of the cadherin cytoplasmic domains with catenin proteins, such as p120-catenin, beta-catenin and alpha-catenin. Catenins, in turn, bind to the actin cytoskeleton. Binding of these proteins to the actin cytoskeleton prevents actin from growing (a process known as polymerization) and therefore keeps cells stationary. Cadherins regulate cell–cell adhesion during development of the body and in adult tissue. Disruption of cadherin proteins, by genetic alteration or by changes to the structure or function of the protein, has been linked to tumor progression. Notably, PTPmu regulates the adhesion of cells to the classical cadherins.[63] PTPmu likely regulates cadherin-dependent adhesion by interacting with both cadherins and catenins via PTPmu’s cytoplasmic domain. To support this assertion, PTPmu has been shown to interact with and/or dephosphorylate many signaling proteins involved in regulating the cadherin-catenin complex, including p120 catenin,[54] and E-cadherin (CDH1 (gene)) and N-cadherin (CDH2).[22][64] PTPmu has also been shown to interact with the c-Met hepatocyte growth factor receptor, a protein that is also localized to adherens junctions.[65] Although p120 catenin is a potential substrate of PTPmu,[54] others have suggested that the interaction between PTPmu and catenins is only indirect through E-cadherin.[66] α3β1 integrin and the tetraspanin CD151 regulate PTPmu gene expression to promote E-cadherin-mediated cell–cell adhesion.[67]
In addition to catenins and cadherins, PTPmu dephosphorylates PIPKIγ90 and nectin-3 (PVRL3) to stabilize E-cadherin-based adherens junctions.[68] PTPmu also dephosphorylates another cell junction protein, connexin 43. The interaction between connexin 43 and PTPmu increases gap junction communication.[69]
Endothelial cell adhesion
[edit]PTPμ is expressed in human umbilical cord vein endothelial cells (HUVEC)[70] and in capillaries in the developing brain.[24] The expression of PTPμ in HUVEC cells increases at higher cell density.[70] Studies of PTPμ expression in animal tissues have demonstrated that PTPμ is preferentially expressed in endothelial cells of arteries and capillaries and in cardiac smooth muscle, in addition to brain cells.[25][26] Because of this specialized expression in arterial endothelial cells, and because PTPμ is found to associate with proteins involved in maintaining endothelial cell–cell junctions, such as VE-cadherin,[71] PTPμ is hypothesized to regulate endothelial cell junction formation or permeability. PTPμ has been shown to be involved in mechanotransduction that results from changes in blood flow to influence endothelial cell-mediated blood vessel dilation, a process induced by “shear stress.”[72] When PTPmu is missing in mice (PTPmu -/- knock-out mice), cannulated mesenteric arteries show reduced flow-induced (or “shear stress” induced) dilation.[72] PTPmu tyrosine phosphatase activity is activated by shear stress.[73] Caveolin 1 is a scaffolding protein enriched in endothelial cell junctions that is also linked to shear stress regulated responses.[73] Caveolin 1 is dephosphorylated on tyrosine 14 in response to shear stress and PTPmu is hypothesized to catalyze this reaction.[73]
Cell migration
[edit]Neurite outgrowth
[edit]PTPmu is expressed in the developing brain and retina.[27][28][29][30][31][74] A brain cell, or neuron, has a cell body that contains the nucleus and two types of extensions or processes that grow out from the cell body, the dendrites and axons. Dendrites generally receive input from other neurons, while axons send output to adjacent neurons. These processes are called neurites when grown ‘’in vitro’’ on tissue culture plates, because it is not clear whether they are dendrites or axons. ‘’In vitro’’ growth studies are useful for evaluating the mechanisms that neurons use to grow and function. A neurite outgrowth assay is a type of experiment where neurons are placed on different adhesive substrates on tissue culture plates. A neurite outgrowth assay is meant to mimic how neurons grow inside the body. During development of the nervous system, neuronal axons reach their often-distant targets by reacting to different substrates in their environment, so-called guidance cues, that are attractive, repulsive or simply permissive, meaning these substrates pull axons toward them, away from them, or act in a way that allows growth, respectively. When PTPmu is applied to a dish as an ‘’in vitro’’ substrate, it promotes neurite outgrowth.[27] PTPmu also acts as a guidance cue during development of the nervous system, by repelling neurites of the temporal neural retina, while permitting growth of neurites from the nasal neural retina.[28] Expression of PTPmu protein capable of dephosphorylating tyrosine residues is required for mediating both nasal neurite outgrowth and temporal neurite repulsion.[75] By blocking the expression of PTPmu protein with antisense technology, or by expressing catalytically inactive mutants of PTPmu (molecules of PTPmu that can not dephosphorylate their target proteins) in the developing retina, it was shown that PTPmu is required for the development of the neural retina.[29]
PTPmu also regulates neurite outgrowth on classical cadherins. PTPmu tyrosine phosphatase activity is necessary for neurite outgrowth on the classical cadherins E-, N- and R-cadherin,[27][60][61] suggesting that PTPmu dephosphorylates key components of the cadherin-catenin complex to regulate axonal migration. Again, this emphasizes that PTPmu likely regulates cadherin-dependent processes via its cytoplasmic domain.
Various signals required for PTPmu-mediated neurite outgrowth and repulsion have been identified. Some of these signals are proteins that interact with, or bind, to PTPmu, whereas, others may be dephosphorylated by PTPmu. PTPmu interacts with the scaffolding proteins RACK1/GNB2L1,[76] and IQGAP1.[77] IQGAP1 is a scaffold for Rho family of GTPases, E-cadherin, beta-catenin and other proteins. IQGAP1 binding to Rho GTPases is necessary for PTPmu-mediated neurite outgrowth.[77] The growing tip of the neuron, the growth cone, has a distinct appearance depending on what signals are activated inside the growth cone when it touches different substrates. The morphology of the growth cones on PTPmu and the repulsion of temporal neurites are both regulated by the Rho GTPase family member, Cdc42.[78][79] Inhibition of the Rho GTPase Rac1 permitted neurite outgrowth on PTPmu from neurons in the temporal retina.[79]
The proteins PLCγ1 (PLCG1), PKCδ (PRKCD) and BCCIP are PTPmu substrates.[80] PKCδ activity is required for PTPmu mediated neurite outgrowth[81] and PTPmu-mediated neurite repulsion.[82] Expression of BCCIP is necessary for PTPmu-mediated neurite outgrowth.[83] PTPmu is cleaved in certain brain cancers, which results in nuclear translocation of the cytoplasmic domain of PTPmu (see below). A possible function for the BCCIP-PTPmu interaction may be to shuttle the intracellular PTPmu fragment into the cell nucleus. In summary, PTPmu dephosphorylates PKCδ, PLCγ1, and BCCIP, and binds to IQGAP1. The expression and/or activity of all these proteins and Cdc42 is necessary for PTPmu-mediated neurite outgrowth. Also, the activity of the GTPase Rac1 promotes PTPmu-mediated neurite repulsion.
Cancer
[edit]PTPmu is downregulated in glioblastoma multiforme (GBM) cells and tissue compared to normal control tissue or cells.[84] The reduction in PTPmu expression in GBM cells has been linked to increased migration of GBM cells.[84][85] [86][87] It was found that PTPmu expression is decreased in GBM cells by proteolysis of the full-length protein into a shed extracellular fragment[88] and a cytoplasmically released intracellular fragment that is capable of translocating into the nucleus.[62] Cleavage of PTPmu is similar to that identified for the Notch signaling pathway. PTPmu is first cleaved to yield two non-covalently associated fragments,[35][53] likely via a furin-like endo-peptidase in the endoplasmic reticulum (ER), as has been demonstrated for another RPTP, LAR (or PTPRF).[89][90] Then PTPmu is likely cleaved by an A disintegrin and metalloproteinase (ADAM) protease in the extracellular domain of PTPmu to release the shed extracellular fragment, then by the gamma secretase complex in the transmembrane domain to release the PTPmu intracellular fragment (reviewed in [20] and [21] Cleavage of PTPmu would likely impact the signaling partners that PTPmu would have access to, as has been proposed. (Phillips-Mason, Craig and Brady-Kalnay, 2011). PLCγ1 is a PTPmu substrate.[80] PLCγ1 activity is necessary for mediating GBM cell migration in the absence of PTPmu,[80] thus it seems likely that PTPmu dephosphorylation of PLCγ1 prevents PLCγ1-mediated migration. Cleavage of cell adhesion molecules, like PTPmu, has also been linked to the deregulation of contact inhibition of growth observed in cancer cells.[20] Visualization of the shed extracellular fragment of PTPmu has been proposed to be an effective means of delineating the borders of a GBM tumor ‘’in vivo.’’[88] Fluorescently tagged PTPmu peptides that bind homophilically to the shed PTPmu extracellular domains are capable of crossing the blood–brain barrier and identifying tumor margins in rodent models of GBM.[88]
Interactions
[edit]PTPRM has been shown to interact with:
- BCCIP,[83]
- c-Met,[65]
- CDH1 E-cadherin (Cadherin-1),[22][64]
- CDH2 N-cadherin (Cadherin-2),[22][64]
- CDH4 R-cadherin (cadherin-4),[64]
- CDH5 VE-cadherin (cadherin 5, CDH5),[71]
- CTNND1 (p120catenin),[54]
- GNB2L1/RACK1,[76]
- GJA1 connexin43 (gap junction protein, alpha 1),[69]
- IQGAP1,[77]
- PVRL3 (nectin3),[68]
- PIPKIγ90,[68]
- PRKCD (PKCδ),[80] and
- PLCG1 (PLCγ1).[80]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000173482 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000033278 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Gebbink MF, van Etten I, Hateboer G, Suijkerbuijk R, Beijersbergen RL, Geurts van Kessel A, Moolenaar WH (Nov 1991). "Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase". FEBS Lett. 290 (1–2): 123–30. doi:10.1016/0014-5793(91)81241-Y. PMID 1655529. S2CID 7237197.
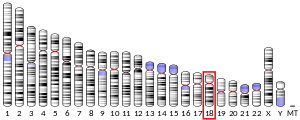
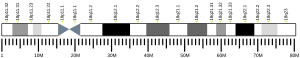
- ^ Suijkerbuijk RF, Gebbink MF, Moolenaar WH, Geurts van Kessel A (Nov 1993). "Fine mapping of the human receptor-like protein tyrosine phosphatase gene (PTPRM) to 18p11.2 by fluorescence in situ hybridization". Cytogenet Cell Genet. 64 (3–4): 245–6. doi:10.1159/000133598. PMID 8404049.
- ^ "Entrez Gene: PTPRM protein tyrosine phosphatase, receptor type, M".
- ^ Tonks NK, Yang Q, Flint AJ, Gebbink MF, Franza BR, Hill DE, Sun H, Brady-Kalnay S (1992). "Protein tyrosine phosphatases: the problems of a growing family" (PDF). Cold Spring Harb Symp Quant Biol. 57: 87–94. doi:10.1101/sqb.1992.057.01.012. PMID 1339708.
- ^ Brady-Kalnay SM, Tonks NK (March 1994). "Protein tyrosine phosphatases: from structure to function". Trends Cell Biol. 4 (3): 73–6. doi:10.1016/0962-8924(94)90172-4. PMID 14731595.
- ^ a b c Brady-Kalnay S (1998). "Ig-superfamily phosphatases". In Peter Sonderegger (ed.). Ig Superfamily Molecules in the Nervous System (6 ed.). Zurich: Harwood Academic Publishers.
- ^ a b c Brady-Kalnay S (2001). "Protein tyrosine phosphatases". In Beckerle, M. (ed.). Cell Adhesion: Frontiers in Molecular Biology (39 ed.). Oxford, UK.: Oxford University Press. pp. 217–258.
- ^ Brady-Kalnay SM, Tonks NK (1995). "Protein tyrosine phosphatases as adhesion receptors". Curr Opin Cell Biol. 7 (5): 650–7. doi:10.1016/0955-0674(95)80106-5. PMID 8573339.
- ^ Brady-Kalnay SM, Tonks NK (1994). "Receptor protein tyrosine phosphatases, cell adhesion and signal transduction". Advances in Protein Phosphatases. 8: 241–71. ISSN 0775-051X.
- ^ a b Bixby JL (March 2001). "Ligands and signaling through receptor-type tyrosine phosphatases". IUBMB Life. 51 (3): 157–63. doi:10.1080/152165401753544223. PMID 11547917. S2CID 44938812.
- ^ Beltran PJ, Bixby JL (January 2003). "Receptor protein tyrosine phosphatases as mediators of cellular adhesion". Front. Biosci. 8 (4): d87–99. doi:10.2741/941. PMID 12456340.
- ^ Johnson KG, Van Vactor D (2003). "Receptor protein tyrosine phosphatases in nervous system development". Physiol Rev. 83 (1): 1–24. doi:10.1152/physrev.00016.2002. PMID 12506125.
- ^ a b Ensslen-Craig SE, Brady-Kalnay SM (2004). "Receptor protein tyrosine phosphatases regulate neural development and axon guidance". Dev Biol. 275 (1): 12–22. doi:10.1016/j.ydbio.2004.08.009. PMID 15464569.
- ^ Burridge K, Sastry SK, Sallee JL (2006). "Regulation of cell adhesion by protein-tyrosine phosphatases. I. Cell–matrix adhesion". J Biol Chem. 281 (23): 15593–6. doi:10.1074/jbc.R500030200. PMID 16497668.
- ^ Sallee JL, Wittchen ES, Burridge K (2006). "Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell–cell adhesion". J Biol Chem. 281 (24): 16189–92. doi:10.1074/jbc.R600003200. PMID 16497667.
- ^ a b c Craig SE, Brady-Kalnay SM (2011). "Cancer cells cut homophilic cell adhesion molecules and run". Cancer Res. 71 (2): 303–9. doi:10.1158/0008-5472.CAN-10-2301. PMC 3343737. PMID 21084269.
- ^ a b Craig SE, Brady-Kalnay SM (2011). "Tumor-derived extracellular fragments of receptor protein tyrosine phosphatases (RPTPs) as cancer molecular diagnostic tools". Anticancer Agents Med Chem. 11 (1): 133–40. doi:10.2174/187152011794941244. PMC 3337336. PMID 21235433.
- ^ a b c d Brady-Kalnay SM, Rimm DL, Tonks NK (1995). "Receptor protein tyrosine phosphatase PTPmu associates with cadherins and catenins in vivo". J Cell Biol. 130 (4): 977–86. doi:10.1083/jcb.130.4.977. PMC 2199947. PMID 7642713.
- ^ Schnekenburger J, Mayerle J, Simon P, Domschke W, Lerch MM (1999). "Protein tyrosine dephosphorylation and the maintenance of cell adhesions in the pancreas". Ann N Y Acad Sci. 880 (1): 157–65. Bibcode:1999NYASA.880..157S. doi:10.1111/j.1749-6632.1999.tb09518.x. PMID 10415859. S2CID 35492083.
- ^ a b Sommer L, Rao M, Anderson DJ (1997). "RPTP delta and the novel protein tyrosine phosphatase RPTP psi are expressed in restricted regions of the developing central nervous system". Dev Dyn. 208 (1): 48–61. doi:10.1002/(SICI)1097-0177(199701)208:1<48::AID-AJA5>3.0.CO;2-1. PMID 8989520.
- ^ a b Bianchi C, Sellke FW, Del Vecchio RL, Tonks NK, Neel BG (1999). "Receptor-type protein-tyrosine phosphatase mu is expressed in specific vascular endothelial beds in vivo". Exp Cell Res. 248 (1): 329–38. doi:10.1006/excr.1999.4428. PMID 10094839.
- ^ a b Koop EA, Lopes SM, Feiken E, Bluyssen HA, van der Valk M, Voest EE, Mummery CL, Moolenaar WH, Gebbink MF (2003). "Receptor protein tyrosine phosphatase mu expression as a marker for endothelial cell heterogeneity; analysis of RPTPmu gene expression using LacZ knock-in mice". Int J Dev Biol. 47 (5): 345–54. PMID 12895029.
- ^ a b c d Burden-Gulley SM, Brady-Kalnay SM (1999). "PTPmu regulates N-cadherin-dependent neurite outgrowth". J Cell Biol. 144 (6): 1323–36. doi:10.1083/jcb.144.6.1323. PMC 2150569. PMID 10087273.
- ^ a b c Burden-Gulley SM, Ensslen SE, Brady-Kalnay SM (2002). "Protein tyrosine phosphatase-mu differentially regulates neurite outgrowth of nasal and temporal neurons in the retina". J Neurosci. 22 (9): 3615–27. doi:10.1523/JNEUROSCI.22-09-03615.2002. PMC 6758368. PMID 11978837.
- ^ a b c Ensslen SE, Rosdahl JA, Brady-Kalnay SM (2003). "The receptor protein tyrosine phosphatase mu, PTPmu, regulates histogenesis of the chick retina". Dev Biol. 264 (1): 106–18. doi:10.1016/j.ydbio.2003.08.009. PMID 14623235.
- ^ a b Chilton JK, Stoker AW (2000). "Expression of receptor protein tyrosine phosphatases in embryonic chick spinal cord". Mol Cell Neurosci. 16 (4): 470–80. doi:10.1006/mcne.2000.0887. PMID 11085882. S2CID 24084590.
- ^ a b Ledig MM, McKinnell IW, Mrsic-Flogel T, Wang J, Alvares C, Mason I, Bixby JL, Mueller BK, Stoker AW (1999). "Expression of receptor tyrosine phosphatases during development of the retinotectal projection of the chick". J Neurobiol. 39 (1): 81–96. doi:10.1002/(SICI)1097-4695(199904)39:1<81::AID-NEU7>3.0.CO;2-K. PMID 10213455.
- ^ Hershman KM, Levitan ES (2000). "RPTPmu and protein tyrosine phosphorylation regulate K(+) channel mRNA expression in adult cardiac myocytes". Am J Physiol Cell Physiol. 278 (2): C397–403. doi:10.1152/ajpcell.2000.278.2.C397. PMID 10666036.
- ^ Brady-Kalnay SM, Flint AJ, Tonks NK (1993). "Homophilic binding of PTP mu, a receptor-type protein tyrosine phosphatase, can mediate cell–cell aggregation". J Cell Biol. 122 (4): 961–72. doi:10.1083/jcb.122.4.961. PMC 2119586. PMID 8394372.
- ^ Gebbink MF, Zondag GC, Wubbolts RW, Beijersbergen RL, van Etten I, Moolenaar WH (1993). "Cell–cell adhesion mediated by a receptor-like protein tyrosine phosphatase". J Biol Chem. 268 (22): 16101–4. doi:10.1016/S0021-9258(19)85392-9. PMID 8393854.
- ^ a b c Brady-Kalnay SM, Tonks NK (1994). "Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTP mu". J Biol Chem. 269 (45): 28472–7. doi:10.1016/S0021-9258(18)46951-7. PMID 7961788.
- ^ a b Del Vecchio RL, Tonks NK (2005). "The conserved immunoglobulin domain controls the subcellular localization of the homophilic adhesion receptor protein-tyrosine phosphatase mu". J Biol Chem. 280 (2): 1603–12. doi:10.1074/jbc.M410181200. PMID 15491993.
- ^ a b Zondag GC, Koningstein GM, Jiang YP, Sap J, Moolenaar WH, Gebbink MF (1995). "Homophilic interactions mediated by receptor tyrosine phosphatases mu and kappa. A critical role for the novel extracellular MAM domain". J Biol Chem. 270 (24): 14247–50. doi:10.1074/jbc.270.24.14247. PMID 7782276.
- ^ Cismasiu VB, Denes SA, Reiländer H, Michel H, Szedlacsek SE (2004). "The MAM (meprin/A5-protein/PTPmu) domain is a homophilic binding site promoting the lateral dimerization of receptor-like protein-tyrosine phosphatase mu". J Biol Chem. 279 (26): 26922–31. doi:10.1074/jbc.M313115200. PMID 15084579.
- ^ a b Aricescu AR, Hon WC, Siebold C, Lu W, van der Merwe PA, Jones EY (2006). "Molecular analysis of receptor protein tyrosine phosphatase mu-mediated cell adhesion". EMBO J. 25 (4): 701–12. doi:10.1038/sj.emboj.7600974. PMC 1383555. PMID 16456543.
- ^ a b Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, van der Merwe PA, Jones EY (2007). "Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism". Science. 317 (5842): 1217–20. Bibcode:2007Sci...317.1217A. doi:10.1126/science.1144646. PMID 17761881. S2CID 15702183.
- ^ Aricescu AR, Siebold C, Jones EY (2008). "Receptor protein tyrosine phosphatase micro: measuring where to stick". Biochem Soc Trans. 36 (Pt 2): 167–72. doi:10.1042/BST0360167. PMID 18363557.
- ^ Becka S, Zhang P, Craig SE, Lodowski DT, Wang Z, Brady-Kalnay SM (2010). "Characterization of the adhesive properties of the type IIb subfamily receptor protein tyrosine phosphatases". Cell Commun Adhes. 17 (2): 34–47. doi:10.3109/15419061.2010.487957. PMC 3337334. PMID 20521994.
- ^ Petrone A, Sap J (2000). "Emerging issues in receptor protein tyrosine phosphatase function: lifting fog or simply shifting?". J Cell Sci. 113 (13): 2345–54. doi:10.1242/jcs.113.13.2345. PMID 10852814.
- ^ a b Hoffmann KM, Tonks NK, Barford D (1997). "The crystal structure of domain 1 of receptor protein-tyrosine phosphatase mu". J Biol Chem. 272 (44): 27505–8. doi:10.1074/jbc.272.44.27505. PMID 9346878.
- ^ Nam HJ, Poy F, Krueger NX, Saito H, Frederick CA (1999). "Crystal structure of the tandem phosphatase domains of RPTP LAR". Cell. 97 (4): 449–57. doi:10.1016/S0092-8674(00)80755-2. PMID 10338209. S2CID 14417598.
- ^ Majeti R, Bilwes AM, Noel JP, Hunter T, Weiss A (1998). "Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge". Science. 279 (5347): 88–91. Bibcode:1998Sci...279...88M. doi:10.1126/science.279.5347.88. PMID 9417031.
- ^ Bilwes AM, den Hertog J, Hunter T, Noel JP (1996). "Structural basis for inhibition of receptor protein-tyrosine phosphatase-alpha by dimerization". Nature. 382 (6591): 555–9. Bibcode:1996Natur.382..555B. doi:10.1038/382555a0. PMID 8700232. S2CID 4233685.
- ^ Meng K, Rodriguez-Peña A, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF (2000). "Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta". Proc Natl Acad Sci U S A. 97 (6): 2603–8. Bibcode:2000PNAS...97.2603M. doi:10.1073/pnas.020487997. PMC 15975. PMID 10706604.
- ^ Blanchetot C, den Hertog J (2000). "Multiple interactions between receptor protein-tyrosine phosphatase (RPTP) alpha and membrane-distal protein-tyrosine phosphatase domains of various RPTPs". J Biol Chem. 275 (17): 12446–52. doi:10.1074/jbc.275.17.12446. PMID 10777529.
- ^ Blanchetot C, Tertoolen LG, Overvoorde J, den Hertog J (2002). "Intra- and intermolecular interactions between intracellular domains of receptor protein-tyrosine phosphatases". J Biol Chem. 277 (49): 47263–9. doi:10.1074/jbc.M205810200. PMID 12376545.
- ^ Gross S, Blanchetot C, Schepens J, Albet S, Lammers R, den Hertog J, Hendriks W (2002). "Multimerization of the protein-tyrosine phosphatase (PTP)-like insulin-dependent diabetes mellitus autoantigens IA-2 and IA-2beta with receptor PTPs (RPTPs). Inhibition of RPTPalpha enzymatic activity". J Biol Chem. 277 (50): 48139–45. doi:10.1074/jbc.M208228200. hdl:2066/185410. PMID 12364328.
- ^ a b c Gebbink MF, Verheijen MH, Zondag GC, van Etten I, Moolenaar WH (1993). "Purification and characterization of the cytoplasmic domain of human receptor-like protein tyrosine phosphatase RPTP mu". Biochemistry. 32 (49): 13516–22. doi:10.1021/bi00212a017. PMID 7504951.
- ^ a b Gebbink MF, Zondag GC, Koningstein GM, Feiken E, Wubbolts RW, Moolenaar WH (1995). "Cell surface expression of receptor protein tyrosine phosphatase RPTP mu is regulated by cell–cell contact". J Cell Biol. 131 (1): 251–60. doi:10.1083/jcb.131.1.251. PMC 2120594. PMID 7559782.
- ^ a b c d Zondag GC, Reynolds AB, Moolenaar WH (2000). "Receptor protein-tyrosine phosphatase RPTPmu binds to and dephosphorylates the catenin p120(ctn)". J Biol Chem. 275 (15): 11264–9. doi:10.1074/jbc.275.15.11264. PMID 10753936.
- ^ a b Groen A, Overvoorde J, van der Wijk T, den Hertog J (2008). "Redox regulation of dimerization of the receptor protein-tyrosine phosphatases RPTPalpha, LAR, RPTPmu and CD45". FEBS J. 275 (10): 2597–604. doi:10.1111/j.1742-4658.2008.06407.x. PMID 18422654. S2CID 199555986.
- ^ Brady-Kalnay SM, Tonks NK (1993). "Purification and characterization of the human protein tyrosine phosphatase, PTP mu, from a baculovirus expression system". Mol Cell Biochem. 127–128: 131–41. doi:10.1007/BF01076764. PMID 7935345. S2CID 24662451.
- ^ Feiken E, van Etten I, Gebbink MF, Moolenaar WH, Zondag GC (2000). "Intramolecular interactions between the juxtamembrane domain and phosphatase domains of receptor protein-tyrosine phosphatase RPTPmu. Regulation of catalytic activity". J Biol Chem. 275 (20): 15350–6. doi:10.1074/jbc.275.20.15350. PMID 10809770.
- ^ Aricescu AR, Fulga TA, Cismasiu V, Goody RS, Szedlacsek SE (2001). "Intramolecular interactions in protein tyrosine phosphatase RPTPmu: kinetic evidence". Biochem Biophys Res Commun. 280 (1): 319–27. doi:10.1006/bbrc.2000.4094. PMID 11162517.
- ^ a b Xie Y, Massa SM, Ensslen-Craig SE, Major DL, Yang T, Tisi MA, Derevyanny VD, Runge WO, Mehta BP, Moore LA, Brady-Kalnay SM, Longo FM (2006). "Protein-tyrosine phosphatase (PTP) wedge domain peptides: a novel approach for inhibition of PTP function and augmentation of protein-tyrosine kinase function". J Biol Chem. 281 (24): 16482–92. doi:10.1074/jbc.M603131200. PMID 16613844.
- ^ a b Oblander SA, Ensslen-Craig SE, Longo FM, Brady-Kalnay SM (2007). "E-cadherin promotes retinal ganglion cell neurite outgrowth in a protein tyrosine phosphatase-mu-dependent manner". Mol Cell Neurosci. 34 (3): 481–92. doi:10.1016/j.mcn.2006.12.002. PMC 1853338. PMID 17276081.
- ^ a b Oblander SA, Brady-Kalnay SM (2010). "Distinct PTPmu-associated signaling molecules differentially regulate neurite outgrowth on E-, N-, and R-cadherin". Mol Cell Neurosci. 44 (1): 78–93. doi:10.1016/j.mcn.2010.02.005. PMC 2881835. PMID 20197094.
- ^ a b Burgoyne AM, Phillips-Mason PJ, Burden-Gulley SM, Robinson S, Sloan AE, Miller RH, Brady-Kalnay SM (2009). "Proteolytic cleavage of protein tyrosine phosphatase mu regulates glioblastoma cell migration". Cancer Res. 69 (17): 6960–8. doi:10.1158/0008-5472.CAN-09-0863. PMC 2747800. PMID 19690139.
- ^ Hellberg CB, Burden-Gulley SM, Pietz GE, Brady-Kalnay SM (March 2002). "Expression of the receptor protein-tyrosine phosphatase, PTPmu, restores E-cadherin-dependent adhesion in human prostate carcinoma cells". J. Biol. Chem. 277 (13): 11165–73. doi:10.1074/jbc.M112157200. PMID 11801604.
- ^ a b c d Brady-Kalnay SM, Mourton T, Nixon JP, Pietz GE, Kinch M, Chen H, Brackenbury R, Rimm DL, Del Vecchio RL, Tonks NK (1998). "Dynamic interaction of PTPmu with multiple cadherins in vivo". J Cell Biol. 141 (1): 287–96. doi:10.1083/jcb.141.1.287. PMC 2132733. PMID 9531566.
- ^ a b Hiscox S, Jiang WG (1999). "Association of the HGF/SF receptor, c-met, with the cell-surface adhesion molecule, E-cadherin, and catenins in human tumor cells". Biochem Biophys Res Commun. 261 (2): 406–11. doi:10.1006/bbrc.1999.1002. PMID 10425198.
- ^ Hiscox S, Jiang WG (1998). "Association of PTPmu with catenins in cancer cells: a possible role for E-cadherin". Int J Oncol. 13 (5): 1077–80. doi:10.3892/ijo.13.5.1077. PMID 9772302.
- ^ Chattopadhyay N, Wang Z, Ashman LK, Brady-Kalnay SM, Kreidberg JA (2003). "alpha3beta1 integrin-CD151, a component of the cadherin-catenin complex, regulates PTPmu expression and cell–cell adhesion". J Cell Biol. 163 (6): 1351–62. doi:10.1083/jcb.200306067. PMC 2173722. PMID 14691142.
- ^ a b c Sakamoto Y, Ogita H, Komura H, Takai Y (2008). "Involvement of nectin in inactivation of integrin alpha(v)beta(3) after the establishment of cell–cell adhesion". J Biol Chem. 283 (1): 496–505. doi:10.1074/jbc.M704195200. PMID 17965016.
- ^ a b Giepmans BN, Feiken E, Gebbink MF, Moolenaar WH (2003). "Association of connexin43 with a receptor protein tyrosine phosphatase". Cell Commun Adhes. 10 (4–6): 201–5. doi:10.1080/cac.10.4-6.201.205. PMID 14681016.
- ^ a b Campan M, Yoshizumi M, Seidah NG, Lee ME, Bianchi C, Haber E (1996). "Increased proteolytic processing of protein tyrosine phosphatase mu in confluent vascular endothelial cells: the role of PC5, a member of the subtilisin family". Biochemistry. 35 (12): 3797–802. doi:10.1021/bi952552d. PMID 8620001.
- ^ a b Sui XF, Kiser TD, Hyun SW, Angelini DJ, Del Vecchio RL, Young BA, Hasday JD, Romer LH, Passaniti A, Tonks NK, Goldblum SE (2005). "Receptor protein tyrosine phosphatase micro regulates the paracellular pathway in human lung microvascular endothelia". Am J Pathol. 166 (4): 1247–58. doi:10.1016/s0002-9440(10)62343-7. PMC 1602370. PMID 15793303.
- ^ a b Koop EA, Gebbink MF, Sweeney TE, Mathy MJ, Heijnen HF, Spaan JA, Voest EE, VanBavel E, Peters SL (2005). "Impaired flow-induced dilation in mesenteric resistance arteries from receptor protein tyrosine phosphatase-mu-deficient mice". Am J Physiol Heart Circ Physiol. 288 (3): H1218–23. doi:10.1152/ajpheart.00512.2004. PMID 15706045. S2CID 40391996.
- ^ a b c Shin J, Jo H, Park H (2006). "Caveolin-1 is transiently dephosphorylated by shear stress-activated protein tyrosine phosphatase mu". Biochem Biophys Res Commun. 339 (3): 737–41. doi:10.1016/j.bbrc.2005.11.077. PMID 16325778.
- ^ Fuchs M, Wang H, Ciossek T, Chen Z, Ullrich A (1998). "Differential expression of MAM-subfamily protein tyrosine phosphatases during mouse development". Mech Dev. 70 (1–2): 91–109. doi:10.1016/S0925-4773(97)00179-2. PMID 9510027. S2CID 9560178.
- ^ Ensslen-Craig SE, Brady-Kalnay SM (2005). "PTP mu expression and catalytic activity are required for PTP mu-mediated neurite outgrowth and repulsion". Mol Cell Neurosci. 28 (1): 177–88. doi:10.1016/j.mcn.2004.08.011. PMID 15607952. S2CID 3813261.
- ^ a b Mourton T, Hellberg CB, Burden-Gulley SM, Hinman J, Rhee A, Brady-Kalnay SM (2001). "The PTPmu protein-tyrosine phosphatase binds and recruits the scaffolding protein RACK1 to cell–cell contacts". J Biol Chem. 276 (18): 14896–901. doi:10.1074/jbc.M010823200. PMID 11278757.
- ^ a b c Phillips-Mason PJ, Gates TJ, Major DL, Sacks DB, Brady-Kalnay SM (2006). "The receptor protein-tyrosine phosphatase PTPmu interacts with IQGAP1". J Biol Chem. 281 (8): 4903–10. doi:10.1074/jbc.M506414200. PMID 16380380.
- ^ Rosdahl JA, Ensslen SE, Niedenthal JA, Brady-Kalnay SM (2003). "PTP mu-dependent growth cone rearrangement is regulated by Cdc42". J Neurobiol. 56 (3): 199–208. doi:10.1002/neu.10231. PMID 12884260.
- ^ a b Major DL, Brady-Kalnay SM (2007). "Rho GTPases regulate PTPmu-mediated nasal neurite outgrowth and temporal repulsion of retinal ganglion cell neurons". Mol Cell Neurosci. 34 (3): 453–67. doi:10.1016/j.mcn.2006.11.022. PMC 185529. PMID 17234431.
- ^ a b c d e Phillips-Mason PJ, Kaur H, Burden-Gulley SM, Craig SE, Brady-Kalnay SM (2011). "Identification of phospholipase C gamma1 as a protein tyrosine phosphatase mu substrate that regulates cell migration". J Cell Biochem. 112 (1): 39–48. doi:10.1002/jcb.22710. PMC 3031780. PMID 20506511.
- ^ Rosdahl JA, Mourton TL, Brady-Kalnay SM (2002). "Protein kinase C delta (PKCdelta) is required for protein tyrosine phosphatase mu (PTPmu)-dependent neurite outgrowth". Mol Cell Neurosci. 19 (2): 292–306. doi:10.1006/mcne.2001.1071. PMID 11860281. S2CID 54361970.
- ^ Ensslen SE, Brady-Kalnay SM (2004). "PTPmu signaling via PKCdelta is instructive for retinal ganglion cell guidance". Mol Cell Neurosci. 25 (4): 558–71. doi:10.1016/j.mcn.2003.12.003. PMID 15080886. S2CID 54311542.
- ^ a b Phillips-Mason PJ, Mourton T, Major DL, Brady-Kalnay SM (2008). "BCCIP associates with the receptor protein tyrosine phosphatase PTPmu". J Cell Biochem. 105 (4): 1059–72. doi:10.1002/jcb.21907. PMC 2758318. PMID 18773424.
- ^ a b Burgoyne AM, Palomo JM, Phillips-Mason PJ, Burden-Gulley SM, Major DL, Zaremba A, Robinson S, Sloan AE, Vogelbaum MA, Miller RH, Brady-Kalnay SM (December 2009). "PTPmu suppresses glioma cell migration and dispersal". Neuro-Oncology. 11 (6): 767–78. doi:10.1215/15228517-2009-019. PMC 2802397. PMID 19304959.
- ^ "NIH Researchers Identify Key Factor that Stimulates Brain Cancer Cells to Spread". News Release. National Institutes of Health (NIH). 2009-08-18. Archived from the original on 2011-10-01. Retrieved 2011-07-21.
- ^ Talan J (2 October 2009). "Investigators Close in on Molecular Target for Glioblastoma Multiforme". Neurology Today. 9 (19): 18. doi:10.1097/01.NT.0000363214.03849.0e. S2CID 56680336.
- ^ Seper C (2009-08-18). "First, cure cancer. Second, build an iPhone app". MedCity News. Retrieved 2011-07-21.
- ^ a b c Burden-Gulley SM, Gates TJ, Burgoyne AM, Cutter JL, Lodowski DT, Robinson S, Sloan AE, Miller RH, Basilion JP, Brady-Kalnay SM (2010). "A novel molecular diagnostic of glioblastomas: detection of an extracellular fragment of protein tyrosine phosphatase mu". Neoplasia. 12 (4): 305–16. doi:10.1593/neo.91940. PMC 2847738. PMID 20360941.
- ^ Streuli M, Krueger NX, Ariniello PD, Tang M, Munro JM, Blattler WA, Adler DA, Disteche CM, Saito H (March 1992). "Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region". EMBO J. 11 (3): 897–907. doi:10.1002/j.1460-2075.1992.tb05128.x. PMC 556530. PMID 1547787.
- ^ Yu Q, Lenardo T, Weinberg RA (June 1992). "The N-terminal and C-terminal domains of a receptor tyrosine phosphatase are associated by non-covalent linkage". Oncogene. 7 (6): 1051–7. PMID 1317540.
Further reading
[edit]- Serra-Pagès C, Medley QG, Tang M, Hart A, Streuli M (June 1998). "Liprins, a family of LAR transmembrane protein-tyrosine phosphatase-interacting proteins". J. Biol. Chem. 273 (25): 15611–20. doi:10.1074/jbc.273.25.15611. PMID 9624153.
- Feiken E, van Etten I, Gebbink MF, Moolenaar WH, Zondag GC (May 2000). "Intramolecular interactions between the juxtamembrane domain and phosphatase domains of receptor protein-tyrosine phosphatase RPTPmu. Regulation of catalytic activity". J. Biol. Chem. 275 (20): 15350–6. doi:10.1074/jbc.275.20.15350. PMID 10809770.