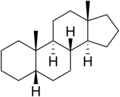
Androstane
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
| Names | |
|---|---|
| IUPAC name 5ξ-Androstane | |
| Systematic IUPAC name (3aS,3bS,5aΞ,9aS,9bS,11aS)-9a,11a-Dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene | |
| Other names Etioallocholane; 10β,13β-Dimethylgonane | |
| Identifiers | |
| |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
PubChem CID | |
| UNII |
|
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C19H32 | |
| Molar mass | 260.465 g·mol−1 |
| Density | 0.95 g/ml |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Androstane is a C19 steroidal hydrocarbon with a gonane core. Androstane can exist as either of two isomers, known as 5α-androstane and 5β-androstane.
- 5α-Androstane
- 5β-Androstane
Pharmacology
[edit]5α-Androstane is reported to be effective as an androgen, in spite of having no oxygen containing functional groups.[1][2]
Androstanes
[edit]Androstanes are steroid derivatives with carbons present at positions 1 through 19.
See also
[edit]References
[edit]- ^ Wilson JD (1996). "Role of dihydrotestosterone in androgen action". Prostate Suppl. 6 (S6): 88–92. doi:10.1002/(SICI)1097-0045(1996)6+<88::AID-PROS17>3.0.CO;2-N. PMID 8630237. S2CID 41352599.
- ^ Segaloff A, Gabbard RB (1960). "5α-Androstane—An Androgenic Hydrocarbon". Endocrinology. 67 (6): 887–889. doi:10.1210/endo-67-6-887. ISSN 0013-7227. PMID 13749674.