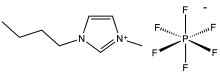
1-Butyl-3-methylimidazolium hexafluorophosphate
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
 | |
| Names | |
|---|---|
| IUPAC name 1-butyl-3-methylimidazol-3-ium hexafluorophosphate | |
| Other names BMIM-PF6 | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.203.179 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C8H15F6N2P | |
| Molar mass | 284.186 g·mol−1 |
| Appearance | Light yellow liquid |
| Density | 1.38 g/mL (20 °C) |
| Melting point | −8 °C (18 °F; 265 K) |
| insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobic and non-water-soluble ionic liquid with a melting point[1] of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMIM-BF4, it is one of the most widely studied ionic liquids. It is known to very slowly decompose in the presence of water.[2]
Preparation
[edit]BMIM-PF6 is commercially available. It may be obtained in two steps: BMIM-Cl is synthesized by alkylating 1-methylimidazole with 1-chlorobutane. A metathesis reaction with potassium hexafluorophosphate gives the desired compound; the tetrafluoroborate may be prepared by analogously using potassium tetrafluoroborate.[3]
See also
[edit]References
[edit]- ^ Mihkel Koel (2008). Ionic Liquids in Chemical Analysis. CRC Press. p. xxvii. ISBN 978-1-4200-4646-5.
- ^ R.P. Swatloski; J.D. Holbrey & R.D. Rogers (2003). "Ionic liquids are not always green: hydrolysis of 1-butyl-3-methylimidazolium hexafluorophosphate". Green Chem. 5 (4): 361–363. doi:10.1039/b304400a.
- ^ Dupont J, Consorti C, Suarez P, de Souza R (2004). "Preparation of 1-Butyl-3-methyl imidazolium-based Room Temperature Ionic Liquids". Organic Syntheses; Collected Volumes, vol. 10, p. 184.
Further reading
[edit]- S. Carda-Broch; A. Berthod; D.W. Armstrong (2003). "Solvent properties of the 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid". Analytical and Bioanalytical Chemistry. 375 (2): 191–199. doi:10.1007/s00216-002-1684-1. PMID 12560962. S2CID 32506513.
