Di-π-methane rearrangement
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
In organic chemistry, the di-π-methane rearrangement is the photochemical rearrangement of a molecule that contains two π-systems separated by a saturated carbon atom. In the aliphatic case, this molecules is a 1,4-diene; in the aromatic case, an allyl-substituted arene. The reaction forms (respectively) an ene- or aryl-substituted cyclopropane. Formally, it amounts to a 1,2 shift of one ene group (in the diene) or the aryl group (in the allyl-aromatic analog), followed by bond formation between the lateral carbons of the non-migrating moiety:[1][2]

Discovery
[edit]This rearrangement was originally encountered in the photolysis of barrelene to give semibullvalene.[3] Once the mechanism was recognized as general by Howard Zimmerman in 1967, it was clear that the structural requirement was two π groups attached to an sp3-hybridized carbon, and then a variety of further examples was obtained.
Notable examples
[edit]
One example was the photolysis of Mariano's compound, 3,3‑dimethyl-1,1,5,5‑tetraphenyl-1,4‑pentadiene. In this symmetric diene, the active π bonds are conjugated to arenes, which does not inhibit the reaction.[4][5][6]

Another was the asymmetric Pratt diene. Pratt's diene demonstrates that the reaction preferentially cyclopropanates aryl substituents, because the reaction pathway preserves the resonant stabilization of a benzhydrylic radical intermediate.[7]
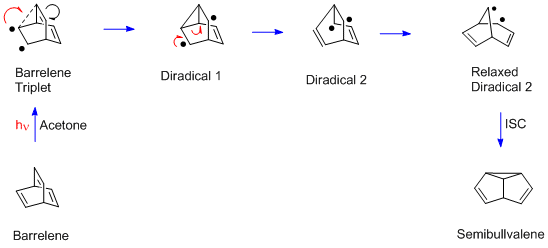
The barrelene rearrangement is more complex than the Mariano and Pratt examples since there are two sp3-hybridized carbons. Each bridgehead carbon has three (ethylenic) π bonds, and any two can undergo the di‑π-methane rearrangement. Moreover, unlike the acyclic Mariano and Pratt dienes, the barrelene reaction requires a triplet excited state. Thus acetone is used in the barrelene reaction; acetone captures the light and then delivers triplet excitation to the barrelene reactant. In the final step of the rearrangement there is a spin flip, to provide paired electrons and a new σ bond.
As excited-state probe
[edit]The dependence of the di-π-methane rearrangement on the multiplicity of the excited state arises from the free-rotor effect.[8] Triplet 1,4-dienes freely undergo cis-trans interconversion of diene double bonds (i.e. free rotation). In acyclic dienes, this free rotation leads to diradical reconnection, short-circuiting the di-π-methane process. Singlet excited states do not rotate and may thus undergo the di-π-methane mechanism. For cyclic dienes, as in the barrelene example, the ring structure can prevent free-rotatory dissipation, and may in fact require bond rotation to complete the rearrangement.
References
[edit]- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "di-π-methane rearrangement". doi:10.1351/goldbook.D01745
- ^ Zimmerman, Howard E.; Armesto, Diego (1996). "Synthetic Aspects of the Di-π-methane Rearrangement". Chemical Reviews. 96 (8): 3065–3112. doi:10.1021/cr910109c. PMID 11848853.
- ^ Zimmerman, H. E.; Grunewald, G. L. (1966). "The Chemistry of Barrelene. III. A Unique Photoisomerization to Semibullvalene". J. Am. Chem. Soc. 88 (1): 183–184. doi:10.1021/ja00953a045.
- ^ Zimmerman, Howard E.; Binkley, Roger W.; Givens, Richard S.; Sherwin, Maynard A. (1967). "Mechanistic organic photochemistry. XXIV. The mechanism of the conversion of barrelene to semibullvalene. A general photochemical process". Journal of the American Chemical Society. 89 (15): 3932–3933. doi:10.1021/ja00991a064. ISSN 0002-7863.
- ^ Zimmerman, H. E.; Mariano, P. S (1969). "The Di-π-methane Rearrangement. Interaction of Electronically Excited Vinyl Chromophores". J. Am. Chem. Soc. 91: 1718–1727. doi:10.1021/ja01035a021.
- ^ Hixson, Stephen S.; Mariano, Patrick S.; Zimmerman, Howard E. (1973). "The Di-π-methane and Oxa-di-π-methane rearrangements". Chemical Reviews. 73 (5): 531. doi:10.1021/cr60285a005.
- ^ Zimmerman, H. E.; Pratt, A. C (1970). "Unsymmetrical Substitution and the Direction of the Di-π-methane Rearrangement; Mechanistic and Exploratory Organic Photochemistry. LVI". J. Am. Chem. Soc. 92: 6259–6267. doi:10.1021/ja00724a026.
- ^ Zimmerman, H. E.; Schissel, D. N (1986). "Di-π-methane Rearrangement of Highly Sterically Congested Molecules: Inhibition of Free Rotor Energy Dissipation. Mechanistic and Exploratory Organic Photochemistry". J. Org. Chem. 51: 196–207. doi:10.1021/jo00352a013.