Lapachol
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
 | |
| Names | |
|---|---|
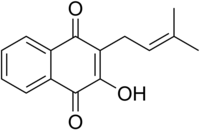
| Preferred IUPAC name 2-Hydroxy-3-(3-methylbut-2-en-1-yl)naphthalene-1,4-dione | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.421 |
| EC Number |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H14O3 | |
| Molar mass | 242.27 |
| Appearance | Yellow crystals |
| Melting point | 140 °C (284 °F; 413 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Lapachol is a natural phenolic compound isolated from the bark of the lapacho tree.[3] This tree is known botanically as Handroanthus impetiginosus, but was formerly known by various other botanical names such as Tabebuia avellanedae.[4] Lapachol is also found in other species of Handroanthus.
Lapachol is usually encountered as a yellow, skin-irritating powder from wood. Chemically, it is a derivative of vitamin K.[5]
Once studied as a possible treatment for some types of cancer, it is now considered too toxic for use.[6][7][8][9]
See also
[edit]§Hooker-Oxidation§
References
[edit]- ^ [1] Lapochol at R&D Chemicals.
- ^ "ChromaDex Incorporated General Product Information for LAPACHOL(RG)". Archived from the original on 2007-09-28. Retrieved 2007-07-06. Lapochol at CromaDex.
- ^ Record, Samuel J.. "Lapachol" pages 17-19. In: Tropical Woods (1925).
- ^ Grose SO, Olmstead RG (2007). "Evolution of a Charismatic Neotropical Clade: Molecular Phylogeny of Tabebuia s.l., Crescentieae, and Allied Genera (Bignoniaceae)". Systematic Botany. 32 (3): 650–659. doi:10.1600/036364407782250553. S2CID 8824926.
- ^ Louis F.Fieser. The Scientific Method pages 163-191. Reinhold Publishing Corporation, New York, 1964
- ^ Felício AC, Chang CV, Brandão MA, Peters VM, Guerra Mde O (2002). "Fetal growth in rats treated with lapachol". Contraception. 66 (4): 289–93. doi:10.1016/S0010-7824(02)00356-6. PMID 12413627.
- ^ Oral toxicology studies with lapachol. Morrison, Robert K.; Brown, Donald Emerson; Oleson, Jerome J.; Cooney, David A. Toxicology and Applied Pharmacology (1970), 17(1), 1-11.
- ^ Guerra Mde O, Mazoni AS, Brandão MA, Peters VM (2001). "Toxicology of Lapachol in rats: embryolethality". Brazilian Journal of Biology. 61 (1): 171–4. doi:10.1590/s0034-71082001000100021. PMID 11340475.
- ^ de Cássia da Silveira E, Sá R, de Oliveira Guerra M (2007). "Reproductive toxicity of lapachol in adult male Wistar rats submitted to short-term treatment". Phytotherapy Research. 21 (7): 658–62. doi:10.1002/ptr.2141. PMID 17421057.