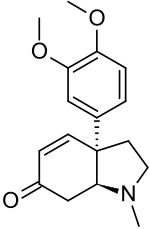
Mesembrenone
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name (3aR,7aS)-3a-(3,4-Dimethoxyphenyl)-1-methyl-1,2,3,3a,7,7a-hexahydro-6H-indol-6-one | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C17H21NO3 | |
| Molar mass | 287.359 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Mesembrenone is an alkaloid constituent of Sceletium tortuosum (Kanna) and minor constituent of Lampranthus aureus and Lampranthus spectabilis. [1]
Similar to modern synthetic antidepressants, it is a potent (IC50 < 1 μM) selective inhibitor of the serotonin transporter (SERT) (that is, a selective serotonin reuptake inhibitor; Ki = 27 nM) and also a phosphodiesterase 4 (PDE4) inhibitor (Ki = 470 nM).[2]
See also
[edit]Other alkaloids present in Kanna include:
References
[edit]- ^ Smith, Michael T.; Field, Courtney R.; Crouch, Neil R.; Hirst, Manton (1998). "The Distribution of Mesembrine Alkaloids in Selected Taxa of the Mesembryanthemaceae and their Modification in the Sceletium Derived 'Kougoed'". Pharmaceutical Biology. 36 (3): 173–179. doi:10.1076/phbi.36.3.173.6350.
- ^ Harvey, A. L.; Young, L. C.; Viljoen, A. M.; Gericke, N. P. (October 2011). "Pharmacological actions of the South African medicinal and functional food plant Sceletium tortuosum and its principal alkaloids". Journal of Ethnopharmacology. 137 (3): 1124–1129. doi:10.1016/j.jep.2011.07.035. PMID 21798331.