Narirutin
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
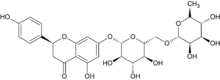 | |
| Names | |
|---|---|
| IUPAC name (2S)-4′,5-Dihydroxy-7-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyloxy]flavan-4-one | |
| Systematic IUPAC name (22S,42S,43R,44S,45S,46R,72R,73R,74R,75R,76S)-14,25,43,44,45,73,74,75-Octahydroxy-76-methyl-22,23-dihydro-24H-3,6-dioxa-2(2,7)-[1]benzopyrana-4(2,6),7(2)-bis(oxana)-1(1)-benzenaheptaphan-24-one | |
| Other names Naringenin-7-O-rutinoside | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.034.655 |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C27H32O14 | |
| Molar mass | 580.539 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Narirutin is a flavanone-7-O-glycoside, consisting of the flavanone naringenin bonded with the disaccharide rutinose.[1]
It is found in orange juice.[1][2]
References
[edit]- ^ a b Rouseff, Russell L.; Martin, Shirley F.; Youtsey, Charles O. (1987). "Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus". Journal of Agricultural and Food Chemistry. 35 (6): 1027–1030. doi:10.1021/jf00078a040. ISSN 0021-8561.
- ^ Widmer W.W and Martin S.F. (1993). "Interferences with naringin and neohesperidin analysis by high performance liquid chromatography".
External links
[edit] Media related to Narirutin at Wikimedia Commons
Media related to Narirutin at Wikimedia Commons