Phosphoramidate
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
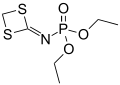
In organophosphorus chemistry, phosphoramidates (sometimes also called amidophosphates) are a class of phosphorus compounds structurally related to phosphates (or organophosphates) via the substitution of an −O− group for an amine group (−N−). They are derivatives of phosphoramidic acids, which possess the structure O=P(OH)(NR2)2 or O=P(OH)2(NR2).
A phosphorodiamidate is a phosphate that has two of its hydroxyl (−OH) groups substituted by amine (NR2) groups to give a species with the general formula O=P(OH)(NH2)2. The substitution of all three OH groups gives the phosphoric triamides (O=P(NR2)3), which are commonly referred to as phosphoramides.[1]
Synthesis
[edit]In the Stokes method, phosphoramidates are synthesized from phosphorus oxychloride. The compound reacts with phenol to form a chlorophosphonate ester or diester, depending on stoichiometry. The remaining chlorine substituents then react with an amine compound to give the phosphoramidate.[2]
Examples
[edit]Two examples of natural phosphoramidates are phosphocreatine and the phosphoramidate formed when histidine residues in histidine kinases are phosphorylated.[3] An example of a phosphorodiamidate is morpholino which is used in molecular biology.
See also
[edit]References
[edit]- ^ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "phosphoramides". doi:10.1351/goldbook.A00484
- ^ Klement, R. (1963). "Phosphorus". In Brauer, Georg (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 1. Translated by Riley, Reed F. (2nd ed.). NY, NY: Academic Press. pp. 579–590. LCCN 63-14307.
- ^ Stock, Ann M.; Robinson, Victoria L.; Goudreau, Paul N. (2000-07-01). "Two-Component Signal Transduction". Annual Review of Biochemistry. 69: 183–215. doi:10.1146/annurev.biochem.69.1.183. PMID 10966457.