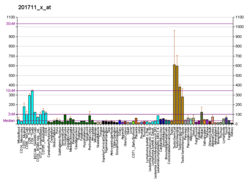
RANBP2
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
RAN binding protein 2 (RANBP2) is protein which in humans is encoded by the RANBP2 gene.[5] It is also known as nucleoporin 358 (Nup358) since it is a member nucleoporin family that makes up the nuclear pore complex. RanBP2 has a mass of 358 kDa.
Function
[edit]RAN is a small GTP-binding protein of the RAS superfamily. Ran GTPase is a master regulatory switch, which among other functions, controls the shuttling of proteins between the nuclear and cytoplasm compartments of the cell. Ran GTPase controls a variety of cellular functions through its interactions with other proteins. The RanBP2 gene encodes a very large RAN-binding protein that localizes to cytoplasmic filaments emanating from the nuclear pore complex. RanBP2/Nup358 is a giant scaffold and mosaic cyclophilin-related nucleoporin implicated in controlling selective processes of the Ran-GTPase cycle. RanBP2 is composed of multiple domains. Each domain of RanBP2 selectively and directly interacts with distinct proteins such as Ran GTPase, importin-beta, exportin-1/CRM1, red opsin, subunits of the proteasome, cox11 and the kinesin-1 isoforms, KIF5B and KIF5C. Another partner of RanBP2 is the E2 enzyme UBC9. RanBP2 strongly enhances SUMO1 transfer from UBC9 to the SUMO1 target SP100. Another target for SUMOylation is RanGAP which is the GTPase activating protein for Ran. SUMO-RanGAP interacts with a domain near the carboxyl terminus of RanBP2. These findings place sumoylation at the cytoplasmic filaments of the nuclear pore complex and suggest that, for some substrates, modification and nuclear import are linked events. The pleiotropic (multifunctional) role of RanBP2 reflects its interaction with multiple partners, each presenting distinct cellular or molecular functions. This gene is partially duplicated in a gene cluster that lies in a hot spot for recombination on human chromosome 2q.
Clinical significance
[edit]Insufficiency of RanBP2 is directly linked to carcinogenesis, aneuploidy, and neuroprotection of photoreceptor neurons to light-elicited stress and aging. Human missense mutations in RanBP2 were identified in its leucine-rich domain and they cause autosomal dominant necrotizing encephalopathy (ADNE).[6]
Interactions
[edit]RANBP2 has been shown to interact with KPNB1[7][8][9] and UBE2I.[10][11]
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000153201 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000003226 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Beddow AL, Richards SA, Orem NR, Macara IG (Apr 1995). "The Ran/TC4 GTPase-binding domain: identification by expression cloning and characterization of a conserved sequence motif". Proceedings of the National Academy of Sciences of the United States of America. 92 (8): 3328–32. Bibcode:1995PNAS...92.3328B. doi:10.1073/pnas.92.8.3328. PMC 42159. PMID 7724562.
- ^ "Entrez Gene: RANBP2 RAN binding protein 2".
- ^ Yaseen NR, Blobel G (Sep 1999). "GTP hydrolysis links initiation and termination of nuclear import on the nucleoporin nup358". The Journal of Biological Chemistry. 274 (37): 26493–502. doi:10.1074/jbc.274.37.26493. PMID 10473610.
- ^ Delphin C, Guan T, Melchior F, Gerace L (Dec 1997). "RanGTP targets p97 to RanBP2, a filamentous protein localized at the cytoplasmic periphery of the nuclear pore complex". Molecular Biology of the Cell. 8 (12): 2379–90. doi:10.1091/mbc.8.12.2379. PMC 25714. PMID 9398662.
- ^ Ben-Efraim I, Gerace L (Jan 2001). "Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import". The Journal of Cell Biology. 152 (2): 411–7. doi:10.1083/jcb.152.2.411. PMC 2199621. PMID 11266456.
- ^ Ewing RM, Chu P, Elisma F, Li H, Taylor P, Climie S, McBroom-Cerajewski L, Robinson MD, O'Connor L, Li M, Taylor R, Dharsee M, Ho Y, Heilbut A, Moore L, Zhang S, Ornatsky O, Bukhman YV, Ethier M, Sheng Y, Vasilescu J, Abu-Farha M, Lambert JP, Duewel HS, Stewart II, Kuehl B, Hogue K, Colwill K, Gladwish K, Muskat B, Kinach R, Adams SL, Moran MF, Morin GB, Topaloglou T, Figeys D (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- ^ Zhang H, Saitoh H, Matunis MJ (Sep 2002). "Enzymes of the SUMO modification pathway localize to filaments of the nuclear pore complex". Molecular and Cellular Biology. 22 (18): 6498–508. doi:10.1128/MCB.22.18.6498-6508.2002. PMC 135644. PMID 12192048.
Further reading
[edit]- Murawala P, Tripathi MM, Vyas P, Salunke A, Joseph J (Sep 2009). "Nup358 interacts with APC and plays a role in cell polarization". Journal of Cell Science. 122 (Pt 17): 3113–22. doi:10.1242/jcs.037523. PMID 19654215. S2CID 263518890.
- Ferreira PA, Hom JT, Pak WL (Sep 1995). "Retina-specifically expressed novel subtypes of bovine cyclophilin". The Journal of Biological Chemistry. 270 (39): 23179–88. doi:10.1074/jbc.270.39.23179. PMID 7559465.
- Yokoyama N, Hayashi N, Seki T, Panté N, Ohba T, Nishii K, Kuma K, Hayashida T, Miyata T, Aebi U (Jul 1995). "A giant nucleopore protein that binds Ran/TC4". Nature. 376 (6536): 184–8. Bibcode:1995Natur.376..184Y. doi:10.1038/376184a0. PMID 7603572. S2CID 4304659.
- Beddow AL, Richards SA, Orem NR, Macara IG (Apr 1995). "The Ran/TC4 GTPase-binding domain: identification by expression cloning and characterization of a conserved sequence motif". Proceedings of the National Academy of Sciences of the United States of America. 92 (8): 3328–32. Bibcode:1995PNAS...92.3328B. doi:10.1073/pnas.92.8.3328. PMC 42159. PMID 7724562.
- Wu J, Matunis MJ, Kraemer D, Blobel G, Coutavas E (Jun 1995). "Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region". The Journal of Biological Chemistry. 270 (23): 14209–13. doi:10.1074/jbc.270.23.14209. PMID 7775481.
- Bischoff FR, Krebber H, Smirnova E, Dong W, Ponstingl H (Feb 1995). "Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1". The EMBO Journal. 14 (4): 705–15. doi:10.1002/j.1460-2075.1995.tb07049.x. PMC 398135. PMID 7882974.
- Ferreira PA, Nakayama TA, Pak WL, Travis GH (Oct 1996). "Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin". Nature. 383 (6601): 637–40. Bibcode:1996Natur.383..637F. doi:10.1038/383637a0. PMID 8857542. S2CID 4304490.
- Mahajan R, Delphin C, Guan T, Gerace L, Melchior F (Jan 1997). "A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2". Cell. 88 (1): 97–107. doi:10.1016/S0092-8674(00)81862-0. PMID 9019411. S2CID 17819277.
- Ferreira PA, Nakayama TA, Travis GH (Feb 1997). "Interconversion of red opsin isoforms by the cyclophilin-related chaperone protein Ran-binding protein 2". Proceedings of the National Academy of Sciences of the United States of America. 94 (4): 1556–61. Bibcode:1997PNAS...94.1556F. doi:10.1073/pnas.94.4.1556. PMC 19830. PMID 9037092.
- Yaseen NR, Blobel G (Apr 1997). "Cloning and characterization of human karyopherin beta3". Proceedings of the National Academy of Sciences of the United States of America. 94 (9): 4451–6. Bibcode:1997PNAS...94.4451Y. doi:10.1073/pnas.94.9.4451. PMC 20743. PMID 9114010.
- Bonifaci N, Moroianu J, Radu A, Blobel G (May 1997). "Karyopherin beta2 mediates nuclear import of a mRNA binding protein". Proceedings of the National Academy of Sciences of the United States of America. 94 (10): 5055–60. Bibcode:1997PNAS...94.5055B. doi:10.1073/pnas.94.10.5055. PMC 24630. PMID 9144189.
- Krebber H, Bastians H, Hoheisel J, Lichter P, Ponstingl H, Joos S (Jul 1997). "Localization of the gene encoding the Ran-binding protein RanBP2 to human chromosome 2q11-q13 by fluorescence in situ hybridization". Genomics. 43 (2): 247–8. doi:10.1006/geno.1997.4777. PMID 9244446.
- Delphin C, Guan T, Melchior F, Gerace L (Dec 1997). "RanGTP targets p97 to RanBP2, a filamentous protein localized at the cytoplasmic periphery of the nuclear pore complex". Molecular Biology of the Cell. 8 (12): 2379–90. doi:10.1091/mbc.8.12.2379. PMC 25714. PMID 9398662.
- Nothwang HG, Rensing C, Kübler M, Denich D, Brandl B, Stubanus M, Haaf T, Kurnit D, Hildebrandt F (Feb 1998). "Identification of a novel Ran binding protein 2 related gene (RANBP2L1) and detection of a gene cluster on human chromosome 2q11-q12". Genomics. 47 (3): 383–92. doi:10.1006/geno.1997.5119. PMID 9480752.
- Ferreira PA, Yunfei C, Schick D, Roepman R (Sep 1998). "The cyclophilin-like domain mediates the association of Ran-binding protein 2 with subunits of the 19 S regulatory complex of the proteasome". The Journal of Biological Chemistry. 273 (38): 24676–82. doi:10.1074/jbc.273.38.24676. hdl:2066/120835. PMID 9733766.
- Vetter IR, Nowak C, Nishimoto T, Kuhlmann J, Wittinghofer A (Mar 1999). "Structure of a Ran-binding domain complexed with Ran bound to a GTP analogue: implications for nuclear transport". Nature. 398 (6722): 39–46. Bibcode:1999Natur.398...39V. doi:10.1038/17969. PMID 10078529. S2CID 4319742.
- Yaseen NR, Blobel G (May 1999). "Two distinct classes of Ran-binding sites on the nucleoporin Nup-358". Proceedings of the National Academy of Sciences of the United States of America. 96 (10): 5516–21. Bibcode:1999PNAS...96.5516Y. doi:10.1073/pnas.96.10.5516. PMC 21891. PMID 10318915.
- Yaseen NR, Blobel G (Sep 1999). "GTP hydrolysis links initiation and termination of nuclear import on the nucleoporin nup358". The Journal of Biological Chemistry. 274 (37): 26493–502. doi:10.1074/jbc.274.37.26493. PMID 10473610.
- Singh BB, Patel HH, Roepman R, Schick D, Ferreira PA (Dec 1999). "The zinc finger cluster domain of RanBP2 is a specific docking site for the nuclear export factor, exportin-1". The Journal of Biological Chemistry. 274 (52): 37370–8. doi:10.1074/jbc.274.52.37370. hdl:2066/120791. PMID 10601307.
- Ben-Efraim I, Gerace L (Jan 2001). "Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import". The Journal of Cell Biology. 152 (2): 411–7. doi:10.1083/jcb.152.2.411. PMC 2199621. PMID 11266456.
- Fauser S, Aslanukov A, Roepman R, Ferreira PA (Jun 2001). "Genomic organization, expression, and localization of murine Ran-binding protein 2 (RanBP2) gene". Mammalian Genome. 12 (6): 406–15. doi:10.1007/s003350010291. PMID 11353387. S2CID 9263393.
- Mavlyutov TA, Cai Y, Ferreira PA (Sep 2002). "Identification of RanBP2- and kinesin-mediated transport pathways with restricted neuronal and subcellular localization". Traffic. 3 (9): 630–40. doi:10.1034/j.1600-0854.2002.30905.x. PMID 12191015. S2CID 26111676.
- Aslanukov A, Bhowmick R, Guruju M, Oswald J, Raz D, Bush RA, Sieving PA, Lu X, Bock CB, Ferreira PA (Oct 2006). "RanBP2 modulates Cox11 and hexokinase I activities and haploinsufficiency of RanBP2 causes deficits in glucose metabolism". PLOS Genetics. 2 (10): e177. doi:10.1371/journal.pgen.0020177. PMC 1626108. PMID 17069463.
- Cho KI, Cai Y, Yi H, Yeh A, Aslanukov A, Ferreira PA (Dec 2007). "Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and function". Traffic. 8 (12): 1722–35. doi:10.1111/j.1600-0854.2007.00647.x. PMID 17887960. S2CID 25829308.
- Yi H, Friedman JL, Ferreira PA (Nov 2007). "The cyclophilin-like domain of Ran-binding protein-2 modulates selectively the activity of the ubiquitin-proteasome system and protein biogenesis". The Journal of Biological Chemistry. 282 (48): 34770–8. doi:10.1074/jbc.M706903200. PMID 17911097.
- Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, Shuai K, Grosschedl R, van Deursen JM (Apr 2008). "Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha". Cell. 133 (1): 103–15. doi:10.1016/j.cell.2008.01.045. PMC 2693193. PMID 18394993.
- Cho KI, Yi H, Yeh A, Tserentsoodol N, Cuadrado L, Searle K, Hao Y, Ferreira PA (Feb 2009). "Haploinsufficiency of RanBP2 is neuroprotective against light-elicited and age-dependent degeneration of photoreceptor neurons". Cell Death and Differentiation. 16 (2): 287–97. doi:10.1038/cdd.2008.153. PMC 2626153. PMID 18949001.
- Cho KI, Yi H, Desai R, Hand AR, Haas AL, Ferreira PA (May 2009). "RANBP2 is an allosteric activator of the conventional kinesin-1 motor protein, KIF5B, in a minimal cell-free system". EMBO Reports. 10 (5): 480–6. doi:10.1038/embor.2009.29. PMC 2680871. PMID 19305391.