Ruthenium hexafluoride
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
| |||
| Names | |||
|---|---|---|---|
| IUPAC name ruthenium(VI) fluoride | |||
| Other names ruthenium(6+) hexafluoride | |||
| Identifiers | |||
3D model (JSmol) | |||
PubChem CID | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| RuF6 | |||
| Molar mass | 215.07 g/mol | ||
| Appearance | dark brown crystalline solid[1] | ||
| Density | 3.54 g/cm3 | ||
| Melting point | 54 °C (129 °F; 327 K)[1] | ||
| Boiling point | 200 °C (392 °F, 473.15 K) (decomposes)[2] | ||
| reacts | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
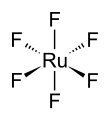
Ruthenium hexafluoride, also ruthenium(VI) fluoride (RuF6), is a compound of ruthenium and fluorine and one of the seventeen known binary hexafluorides.
Synthesis
[edit]Ruthenium hexafluoride is made by a direct reaction of ruthenium metal in a gas stream of fluorine and argon at 400–450 °C. The yields of this reaction are less than 10%.[3]
- Ru + 3 F
2 → RuF
6
Description
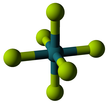
[edit]Ruthenium hexafluoride is a dark brown crystalline solid that melts at 54 °C.[1] The solid structure measured at −140 °C is orthorhombic space group Pnma. Lattice parameters are a = 9.313 Å, b = 8.484 Å, and c = 4.910 Å. There are four formula units (in this case, discrete molecules) per unit cell, giving a density of 3.68 g·cm−3.[3]
The RuF6 molecule itself (the form important for the liquid or gas phase) has octahedral molecular geometry, which has point group (Oh). The Ru–F bond length is 1.818 Å.[3]
References
[edit]- ^ a b c CRC Handbook of Chemistry and Physics, 90th Edition, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 4, Physical Constants of Inorganic Compounds, p. 4-85.
- ^ Haynes, William M (2014-06-04). CRC Handbook of Chemistry and Physics, 95th Edition. CRC Press. ISBN 9781482208689.
- ^ a b c T. Drews, J. Supeł, A. Hagenbach, K. Seppelt: "Solid State Molecular Structures of Transition Metal Hexafluorides", in: Inorganic Chemistry, 2006, 45 (9), S. 3782–3788; doi:10.1021/ic052029f; PMID 16634614.
Further reading
[edit]- Gmelins Handbuch der anorganischen Chemie, System Nr. 63, Ruthenium, Supplement, pp. 266–268.
External links
[edit] Media related to Ruthenium hexafluoride at Wikimedia Commons
Media related to Ruthenium hexafluoride at Wikimedia Commons- Ruthenium hexafluoride at webelements.com.