Tambjamine
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 Biosynthetic precursor to the tambjamines | |
| Names | |
|---|---|
| IUPAC name 4-Methoxy-2,2'-bipyrrole-5-carboxaldehyde | |
| Other names MBC | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| |
| |
| Properties | |
| C10H10N2O2 | |
| Molar mass | 190.202 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tambjamines are a group of natural products that are structurally related to the prodiginines. They are enamine derivatives of 4-methoxy-2,2'-bipyrrole-5-carboxaldehyde (MBC).[1]
Chemical structure
[edit]Tambjamines are composed of two pyrrole rings with an enamine moiety at C-5 and a methoxy group at C-4: the majority have short alkyl chains connected to the enamine nitrogen.[2] This group of alkaloids have been isolated from marine invertebrates and bacteria (both marine and terrestrial).
- Structures of some tambjamines
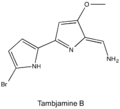
- Tambjamine A
- Tambjamine B
- Tambjamine C
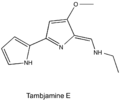
- Tambjamine E
- Tambjamine K
Marine sources and ecological roles
[edit]The large nudibranch Roboastra tigris is a known predator of Tambja eliora and Tambja abdere, two species of smaller nudibranchs. The chemical extracts of all three nudibranch species contain tambjamines, which were traced to Sessibugula translucens, a food source of the two prey species. It is hypothesized that tambjamines are a chemical defence mechanism of the bryozoan against feeding by the spotted kelpfish Gibbonsia elegans.[3][4]
Production
[edit]Biosynthesis
[edit]
The biosynthetic gene cluster responsible for tambjamine production was identified in 2007 using functional genomic analysis of a Pseudoalteromonas tunicata strain. The Tam cluster consists of 19 proteins, 12 of which were found to be highly similar to proteins in the Red and Pig pathways from prodigiosin biosynthesis, based on sequence data. The biosynthesis of tambjamine YP1 first involves the incorporation of proline, malonyl Co-A, and serine to form 4-methoxy-2,2'-bipyrrole-5-carboxaldehyde (MBC).
AfaA is hypothesized to activate long-chain fatty acids while the predicted dehydrogenase, TamT, introduces a double bond into a fatty acyl side chain. TamH then carries out the reduction of the CoA-ester to form an aldehyde intermediate, followed by transamination. Condensation of the dodec-3-en-1-amine product of this reaction and MBC by TamQ, results in the tambjamine YP1 (compound 21 in Figure 1).[5][6]
Laboratory
[edit]The aldehyde MBC was first prepared by total synthesis when the structure of prodigiosin was being investigated.[7] It has subsequently been synthesised by other methods and used to make tambjamines and related natural products.[8]
See also
[edit]References
[edit]- ^ Hernández, Paulina Iglesias; Moreno, Daniel; Javier, Anatalia Araujo; Torroba, Tomás; Pérez-Tomás, Ricardo; Quesada, Roberto (2012). "Tambjamine alkaloids and related synthetic analogs: Efficient transmembrane anion transporters". Chem. Commun. 48 (10): 1556–1558. doi:10.1039/c1cc11300c. PMID 21528145.
- ^ Carbone, Marianna; Irace, Carlo; Costagliola, Francesca; Castelluccio, Francesco; Villani, Guido; Calado, Gonçalo; Padula, Vinicius; Cimino, Guido; Lucas Cervera, J.; Santamaria, Rita; Gavagnin, Margherita (2010). "A new cytotoxic tambjamine alkaloid from the Azorean nudibranch Tambja ceutae". Bioorganic & Medicinal Chemistry Letters. 20 (8): 2668–2670. doi:10.1016/j.bmcl.2010.02.020. PMID 20227875.
- ^ Carté, B.; Faulkner, D. J. (1983). "Defensive metabolites from three nembrothid nudibranchs". The Journal of Organic Chemistry. 48 (14): 2314. doi:10.1021/jo00162a003.
- ^ Carté, Brad; Faulkner, D. John (1986). "Role of secondary metabolites in feeding associations between a predatory nudibranch, two grazing nudibranchs, and a bryozoan". Journal of Chemical Ecology. 12 (3): 795–804. Bibcode:1986JCEco..12..795C. doi:10.1007/BF01012111. PMID 24306917. S2CID 20245013.
- ^ a b Sakai-Kawada, Francis E.; Ip, Courtney G.; Hagiwara, Kehau A.; Awaya, Jonathan D. (2019). "Biosynthesis and Bioactivity of Prodiginine Analogs in Marine Bacteria, Pseudoalteromonas: A Mini Review". Frontiers in Microbiology. 10: 1715. doi:10.3389/fmicb.2019.01715. PMC 6667630. PMID 31396200.
- ^ Burke, Catherine; Thomas, Torsten; Egan, Suhelen; Kjelleberg, Staffan (2007). "The use of functional genomics for the identification of a gene cluster encoding for the biosynthesis of an antifungal tambjamine in the marine bacterium Pseudoalteromonas tunicata". Environmental Microbiology. 9 (3): 814–818. Bibcode:2007EnvMi...9..814B. doi:10.1111/j.1462-2920.2006.01177.x. PMID 17298379.
- ^ Rapoport, Henry.; Willson, Clyde D. (1962). "The Preparation and Properties of Some Methoxypyrroles". Journal of the American Chemical Society. 84 (4): 630–635. doi:10.1021/ja00863a025.
- ^ Hu, Dennis X.; Withall, David M.; Challis, Gregory L.; Thomson, Regan J. (2016). "Structure, Chemical Synthesis, and Biosynthesis of Prodiginine Natural Products". Chemical Reviews. 116 (14): 7818–7853. doi:10.1021/acs.chemrev.6b00024. PMC 5555159. PMID 27314508.