Tetrazole
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
| |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.005.477 | ||
PubChem CID | |||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| CH2N4 | |||
| Molar mass | 70.05 g/mol | ||
| Density | 1.477 g/mL | ||
| Melting point | 157 to 158 °C (315 to 316 °F; 430 to 431 K)[2] | ||
| Boiling point | 220 ± 23 °C (428 ± 41 °F; 493 ± 23 K) | ||
| Acidity (pKa) | 4.90[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Tetrazoles are a class of synthetic organic heterocyclic compound, consisting of a 5-member ring of four nitrogen atoms and one carbon atom. The name tetrazole also refers to the parent compound with formula CH2N4, of which three isomers can be formulated.
Structure and bonding
[edit]Three isomers of the parent tetrazole exist, differing in the position of the double bonds: 1H-, 2H-, and 5H-tetrazole. The 1H- and 2H- isomers are tautomers, with the equilibrium lying on the side of 1H-tetrazole in the solid phase.[3][4][5] In the gas phase, 2H-tetrazole dominates.[4][6][7] These isomers can be regarded as aromatic, with 6 π-electrons, while the 5H-isomer is nonaromatic.
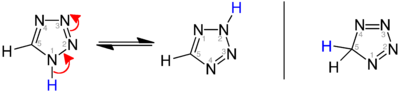
Phosphorus analogs do not have the same electronic nature, with 1H-tetraphosphole having a more pyramidal geometry of the phosphorus at position 1. Instead, it is the anionic tetraphospholides that are aromatic.[8]
Strongly inductively electron-withdrawing functional groups attached to a tetrazole may stabilize a tautomeric ring-opening equilibrium with an azidoimine form.[9]
Synthesis
[edit]1H-Tetrazole was first prepared by the reaction of anhydrous hydrazoic acid and hydrogen cyanide under pressure. Treatment of organic nitriles with sodium azide in the presence of iodine or silica-supported sodium bisulfate as a heterogeneous catalyst enables an advantageous synthesis of 5-substituted 1H-tetrazoles. Another method is the deamination of 5-aminotetrazole, which can be commercially obtained or prepared in turn from aminoguanidine.[10][11]
2-Aryl-2H-tetrazoles are synthesized by a [3+2] cycloaddition reaction between an aryl diazonium and trimethylsilyldiazomethane.[12]
Uses
[edit]There are several pharmaceutical agents which are tetrazoles, including several cephalosporin-class antibiotics. Tetrazoles can act as bioisosteres for carboxylate groups because they have similar pKa and are deprotonated at physiological pH. Angiotensin II receptor blockers — such as losartan and candesartan, often are tetrazoles. A well-known tetrazole is dimethyl thiazolyl diphenyl tetrazolium bromide (MTT). This tetrazole is used in the MTT assay to quantify the respiratory activity of live cells culture, although it generally kills the cells in the process. Some tetrazoles can also be used in DNA assays.[13] Studies suggest VT-1161 and VT-1129 are a potential potent antifungal drugs as they disturbs fungal enzymatic function but not human enzymes.[14][15]
Some tetrazole derivatives with high energy have been investigated as high performance explosives as a replacement for TNT and also for use in high performance solid rocket propellant formulations.[16][17] These include the azidotetrazolate salts of nitrogen bases.
Other tetrazoles are used for their explosive or combustive properties, such as tetrazole itself and 5-aminotetrazole, which are sometimes used as a component of gas generators in automobile airbags. Tetrazole based energetic materials produce high-temperature, non-toxic reaction products such as water and nitrogen gas,[18] and have a high burn rate and relative stability,[19] all of which are desirable properties. The delocalization energy in tetrazole is 209 kJ/mol.
1H-Tetrazole and 5-(benzylthio)-1H-tetrazole (BTT) are widely used as acidic activators of the coupling reaction in oligonucleotide synthesis.[20]
2-Tetrazoles can undergo controlled thermal decomposition to form highly reactive nitrilimines.[21][22] These can in turn undergo a variety of 1,3-dipolar cycloaddition reactions.[23]

Related heterocycles
[edit]- Triazoles, analogs with three nitrogen atoms
- Pentazole, the analog with five nitrogen atoms (strictly speaking, an inorganic homocycle, not a heterocycle)
- Oxatetrazole
- Thiatetrazole
References
[edit]- ^ Satchell, Jacqueline F.; Smith, Brian J. (2002). "Calculation of aqueous dissociation constants of 1,2,4-triazole and tetrazole: A comparison of solvation models". Phys. Chem. Chem. Phys. 4 (18): 4314–4318. Bibcode:2002PCCP....4.4314S. doi:10.1039/b203118c.
- ^ Mihina, Joseph S.; Herbst, Robert M. (1950). "The Reaction of Nitriles with Hydrazoic Acid: Synthesis of Monosubstituted Tetrazoles". J. Org. Chem. 15 (5): 1082–1092. doi:10.1021/jo01151a027.
- ^ Goddard, R.; Heinemann, O.; Krüger, C. (1997-05-15). "α-1H-1,2,3,4-Tetrazole". Acta Crystallographica Section C. 53 (5): 590–592. Bibcode:1997AcCrC..53..590G. doi:10.1107/S0108270197000772. ISSN 0108-2701.
- ^ a b Kiselev, Vitaly G.; Cheblakov, Pavel B.; Gritsan, Nina P. (2011-03-10). "Tautomerism and Thermal Decomposition of Tetrazole: High-Level ab Initio Study". The Journal of Physical Chemistry A. 115 (9): 1743–1753. Bibcode:2011JPCA..115.1743K. doi:10.1021/jp112374t. ISSN 1089-5639. PMID 21322546.
- ^ Razynska, A.; Tempczyk, A.; Malinski, E.; Szafranek, J.; Grzonka, Z.; Hermann, P.: in J. Chem. Soc. Perkin Trans. 2 1983, 379.
- ^ Wong, Ming Wah; Leung-Toung, Regis; Wentrup, Curt (1993-03-01). "Tautomeric equilibrium and hydrogen shifts of tetrazole in the gas phase and in solution". Journal of the American Chemical Society. 115 (6): 2465–2472. doi:10.1021/ja00059a048. ISSN 0002-7863.
- ^ Rażyńska, Anna; Tempczyk, Anna; Maliński, Edmund; Szafranek, Janusz; Grzonka, Zbigniew; Hermann, Peter (1983-01-01). "Application of mass spectrometry to the study of prototropic equilibria in 5-substituted tetrazoles in the gas phase; experimental evidence and theoretical considerations". Journal of the Chemical Society, Perkin Transactions 2 (3): 379–383. doi:10.1039/P29830000379. ISSN 1364-5471.
- ^ Collier, S. J. (2004). "Product Class 24: Tetraphospholes". In Storr, R. C.; Gilchrist, T. L. (eds.). Science of Synthesis. Vol. 13: Category 2, Hetarenes and Related Ring Systems. Thieme. doi:10.1055/sos-SD-013-01194. ISBN 978-3-13-112281-0.
- ^ Burke, Luke A. (25 April 1983). "Possible cause for 5-trichloromethyltetrazole explosion" (letter to the editor), Chemical & Engineering News. p. 2. doi:10.1021/cen-v061n017.p002; but see Beck, Wolfgang and Geisenberger, Josef (5 Mar 1984). "5-Trichloromethyltetrazole", Ibid. p. 39. doi:10.1021/cen-v062n010.p002, which indicates that the trichloromethyl derivative does not exhibit such an equilibrium.
- ^ Henry, Ronald A.; Finnegan, William G. (1954-01-01). "An Improved Procedure for the Deamination of 5-Aminotetrazole". Journal of the American Chemical Society. 76 (1): 290–291. doi:10.1021/ja01630a086. ISSN 0002-7863.
- ^ Kurzer, F.; Godfrey, L. E. A. (1963). "Syntheses of Heterocyclic Compounds from Aminoguanidine". Angewandte Chemie International Edition in English. 2 (8): 459–476. doi:10.1002/anie.196304591. ISSN 1521-3773.
- ^ Patouret, Remi; Kamenecka, Theodore M. (2016-04-06). "Synthesis of 2-aryl-2H-tetrazoles via a regioselective [3+2] cycloaddition reaction". Tetrahedron Letters. 57 (14): 1597–1599. doi:10.1016/j.tetlet.2016.02.102. PMC 4810784. PMID 27041776.
- ^ S Berner; K Mühlegger & H Seliger (Feb 11, 1989). "Studies on the role of tetrazole in the activation of phosphoramidites". Nucleic Acids Res. 17 (3): 853–864. doi:10.1093/nar/17.3.853. PMC 331708. PMID 2922273.
- ^ Warrilow, A. G. S.; Hull, C. M.; Parker, J. E.; Garvey, E. P.; Hoekstra, W. J.; Moore, W. R.; Schotzinger, R. J.; Kelly, D. E.; Kelly, S. L. (December 2014). "The Clinical Candidate VT-1161 Is a Highly Potent Inhibitor of Candida albicans CYP51 but Fails To Bind the Human Enzyme". Antimicrobial Agents and Chemotherapy. 58 (12): 7121–7127. doi:10.1128/AAC.03707-14. PMC 4249504. PMID 25224009.
- ^ Lockhart, Shawn R.; Fothergill, Annette W.; Iqbal, Naureen; Bolden, Carol B.; Grossman, Nina T.; Garvey, Edward P.; Brand, Stephen R.; Hoekstra, William J.; Schotzinger, Robert J.; Ottinger, Elizabeth; Patterson, Thomas F.; Wiederhold, Nathan P. (April 2016). "The Investigational Fungal Cyp51 Inhibitor VT-1129 Demonstrates Potent Activity against Cryptococcus neoformans and Cryptococcus gattii". Antimicrobial Agents and Chemotherapy. 60 (4): 2528–2531. doi:10.1128/AAC.02770-15. PMC 4808209. PMID 26787697.
- ^ "Greener explosives show promise". Chemistry World. 2 October 2008.
- ^ Niko Fischer; Konstantin Karaghiosoff; Thomas M. Klapötke; Jörg Stierstorfer (April 2010). "New Energetic Materials featuring Tetrazoles and Nitramines – Synthesis, Characterization and Properties". Zeitschrift für Anorganische und Allgemeine Chemie. 636 (5): 735–749. doi:10.1002/zaac.200900521.
- ^ Tore Brinck, Thomas M. Klapötke and Jörg Stierstorfer (2014). "Energetic Tetrazole N-oxides". Energetic Tetrazole N -oxides. pp. 133–178. doi:10.1002/9781118676448.ch06. ISBN 9781118676448.
{{cite book}}:|journal=ignored (help) - ^ Nicholas Piekiel & Michael R. Zachariah (2012). "Decomposition of Aminotetrazole Based Energetic Materials under High Heating Rate Conditions". J. Phys. Chem. A. 116 (6): 1519–1526. Bibcode:2012JPCA..116.1519P. doi:10.1021/jp203957t. PMID 22214278.
- ^ Xia Wei (May 6, 2013). "Coupling activators for the oligonucleotide synthesis via phosphoramidite approach". Tetrahedron. 69 (18): 3615–3637. doi:10.1016/j.tet.2013.03.001.
- ^ Huisgen, Rolf; Seidel, Michael; Sauer, Juergen; McFarland, James; Wallbillich, Guenter (June 1959). "Communications: The Formation of Nitrile Imines in the Thermal Breakdown of 2,5-Disubstituted Tetrazoles". The Journal of Organic Chemistry. 24 (6): 892–893. doi:10.1021/jo01088a034.
- ^ Bertrand, Guy; Wentrup, Curt (17 March 1994). "Nitrile Imines: From Matrix Characterization to Stable Compounds". Angewandte Chemie International Edition in English. 33 (5): 527–545. doi:10.1002/anie.199405271.
- ^ Huisgen, Rolf (October 1963). "1,3-Dipolar Cycloadditions. Past and Future". Angewandte Chemie International Edition in English. 2 (10): 565–598. doi:10.1002/anie.196305651.


