Transition metal thiolate complex
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia

Transition metal thiolate complexes are metal complexes containing thiolate ligands. Thiolates are ligands that can be classified as soft Lewis bases. Therefore, thiolate ligands coordinate most strongly to metals that behave as soft Lewis acids as opposed to those that behave as hard Lewis acids. Most complexes contain other ligands in addition to thiolate, but many homoleptic complexes are known with only thiolate ligands. The amino acid cysteine has a thiol functional group, consequently many cofactors in proteins and enzymes feature cysteinate-metal cofactors.[2]
Thiolate as a ligand
[edit]Thiolate is classified as an X ligand in the Covalent bond classification method. In the usual electron counting method, it is a one-electron ligand when terminal and a three-electron ligand when doubly bridging. From the electric structure perspective, thiolate is a pi-donor ligand, akin to alkoxide. One consequence is that few polythiolate complexes are low spin. Another consequence is that electron-precise thiolate complexes tend to be rather nucleophilic. From the perspective of HSAB theory, thiolates are soft. Late metal thiolates are water stable but early metal thiolates tend to hydrolyze. For example, Mo(t-BuS)4, a dark red diamagnetic solid, is sensitive to oxygen and moisture.[3]
Monothiolate ligands range from simple, nonbulky methanethiolate (CH3S−) to very bulky arylthiolates. Many dithiolate ligands are known, starting with the conjugate bases of 1,2-ethanedithiol and 1,3-propanedithiol. Unsaturated versions of 1,2-dithiolates usually take the form of dithiolene ligands, the parent member being (HC)2S22-. 3)2]2. A weak Fe-C(ipso) bond is indicated by the Fe---C distance of 2.427(1) Å. The structure illustrates the low coordination numbers enabled by bulky ligands.[1]
Synthesis
[edit]Metal thiolate complexes are commonly prepared by reactions of metal complexes with thiols (RSH), thiolates (RS−), and disulfides (R2S2). The salt metathesis reaction route is common. In this method, an alkali metal thiolate is treated with a transition metal halide to produce an alkali metal halide and the metal thiolate complex. For example, lithium tert-butylthiolate reacts with MoCl4 to give the tetrathiolate complex:[3]
- MoCl4 + 4 t-BuSLi → Mo(t-BuS)4 + 4 LiCl
Nickelocene and ethanethiol react to give a dimeric thiolate, one cyclopentadienyl ligand serving as a base:
- 2 HSC2H5 + 2 Ni(C5H5)2 → [Ni(SC2H5)(C5H5)]2 + 2 C5H6
Regarding their mechanism of formation from thiols, metal thiolate complexes can arise via deprotonation of thiol complexes.[4][5]
Redox routes
[edit]
Many thiolate complexes are prepared by redox reactions. Organic disulfides oxidize low valence metals, as illustrated by the oxidation of titanocene dicarbonyl:
- (C5H5)2Ti(CO)2 + (C6H5S)2 → (C5H5)2Ti(SC6H5)2 + 2 CO
Some metal centers are oxidized by thiols, the coproduct being hydrogen gas:
- Fe3(CO)12 + 2 C2H5SH → Fe2(SC2H5)2(CO)6 + Fe(CO)5 + CO + H2
These reactions may proceed by the oxidative addition of the thiol to Fe(0).
Thiols and especially thiolate salts are reducing agents. Consequently, they induce redox reactions with certain transition metals. This phenomenon is illustrated by the synthesis of cuprous thiolates from cupric precursors:
- 4 HSC6H5 + 2 CuO → 2 Cu(SC6H5) + (C6H5S)2 + 2 H2O
Thiolate clusters of the type [Fe4S4(SR)4]2− occur in iron–sulfur proteins. Synthetic analogues can be prepared by combined redox and salt metathesis reactions:[6]
- 4 FeCl3 + 6 NaSR + 6 NaSH → Na2[Fe4S4(SR)4] + 10 NaCl + 4 HCl + H2S + R2S2
Reactions
[edit]Thiolates are relatively basic ligands, being derived from conjugate acids with pKa's of 6.5 (thiophenol) to 10.5 (butanethiol). Consequently, thiolate ligand often bridge pairs of metals. One example is Fe2(SCH3)2(CO)6. Thiolate ligands, especially when nonbridging, are susceptible to attack by electrophiles including acids, alkylating agents, and oxidants.
Occurrence and applications
[edit]- Metal thiolates in bioinorganic chemistry
- The zinc finger motif, which is found in transcription factors.
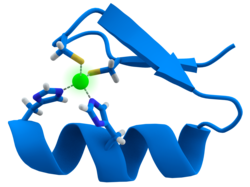
- Structure of [Fe4S4(SMe)4]2−, a synthetic analogue of 4Fe-4S cofactors.[7]
- Thiomersal, a disinfectant, is a metal thiolate complex containing Hg(II)
Metal thiolate functionality, almost always provided by the cysteine residue, is pervasive in metalloenzymes. Iron-sulfur proteins, blue copper proteins, and the zinc-containing enzyme liver alcohol dehydrogenase feature thiolate ligands. The pi-donor property of thiolate ligands stabilizes Fe(IV) states in the enzyme cytochrome P450. All molybdoproteins feature thiolates in the form of cysteinyl and/or molybdopterin.[8] Metallothionins are cysteine-rich proteins that bind heavy metals.
References
[edit]- ^ a b Nguyen, T.; Panda, A.; Olmstead, M. M.; Richards, A. F.; Stender, M.; Brynda, M.; Power, P. P. (2005). "Synthesis and Characterization of Quasi-Two-Coordinate Transition Metal Dithiolates M(SAr)2 (M = Cr, Mn, Fe, Co, Ni, Zn; Ar = C6H3-2,6(C6H2-2,4,6-Pri3)2)". J. Am. Chem. Soc. 127 (23): 8545–8552. doi:10.1021/ja042958q. PMID 15941290.
- ^ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ^ a b Otsuka, Sei; Kamata, Masato; Hirotsu, Ken; Higuchi, Taiichi (1981). "A Novel Molybdenum Thiolato Compound, Tetrakis(tert-butylthiolato)molybdenum(IV). Preparation and Crystal and Molecular Structure". Journal of the American Chemical Society. 103 (11): 3011–3014. doi:10.1021/ja00401a017.
- ^ Jessop, Philip G.; Morris, Robert H. (1993). "Hydrogen/Deuterium exchange reactions of an iridium dithiol complex". Inorganic Chemistry. 32 (11): 2236–2237. doi:10.1021/ic00063a006.
- ^ Heller, M.; Sheldrick, W. S. (2004). "Copper(I) Coordination Polymers with Alkanedithiol and -dinitrile Bridging Ligands". Zeitschrift für anorganische und allgemeine Chemie. 630 (12): 1869–1874. doi:10.1002/zaac.200400165.
- ^ Lee, S. C.; Lo, W.; Holm, R. H. (2014). "Developments in the Biomimetic Chemistry of Cubane-Type and Higher Nuclearity Iron–Sulfur Clusters". Chemical Reviews. 114 (7): 3579–3600. doi:10.1021/cr4004067. PMC 3982595. PMID 24410527.
- ^ Axel Kern; Christian Näther; Felix Studt; Felix Tuczek (2004). "Substitution of Sulfur by Selenium in the Series [Fe4X4(YCH3)4]2-; X = S/Se and Y = S/Se". Inorg. Chem. 43 (16): 5003–5010. doi:10.1021/ic030347d. PMID 15285677.
- ^ S. J. Lippard, J. M. Berg "Principles of Bioinorganic Chemistry" University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.

![Structure of [Fe4S4(SMe)4]2−, a synthetic analogue of 4Fe-4S cofactors.[7]](http://upload.wikimedia.org/wikipedia/commons/thumb/9/92/OBINIX2.png/250px-OBINIX2.png)

