White phosphorus
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 White phosphorus sample with a chunk removed from the corner to expose un-oxidized material | |
 Tetraphosphorus molecule | |
| Names | |
|---|---|
| IUPAC names White phosphorus Tetraphosphorus | |
| Systematic IUPAC name 1,2,3,4-Tetraphosphatricyclo[1.1.0.02,4]butane | |
Other names
| |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.107.967 |
| 1856 | |
PubChem CID | |
| UN number | 1381 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| P4 | |
| Molar mass | 123.895 g·mol−1 |
| Density | 1.82 g/cm3 |
| Melting point | 44.1 °C; 111.4 °F; 317.3 K |
| Boiling point | 280 °C; 536 °F; 553 K |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is one of allotropes of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus),[1] and impure white phosphorus is for this reason called yellow phosphorus. White phosphorus is the first allotrope of phosphorus, and in fact the first elementary substance to be discovered that was not known since ancient times.[2] It glows greenish in the dark (when exposed to oxygen) and is highly flammable and pyrophoric (self-igniting) upon contact with air. It is toxic, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation. The odour of combustion of this form has a characteristic garlic odor, and samples are commonly coated with white "diphosphorus pentoxide", which consists of P4O10 tetrahedra with oxygen inserted between the phosphorus atoms and at their vertices. White phosphorus is only slightly soluble in water and can be stored under water. P4 is soluble in benzene, oils, carbon disulfide, and disulfur dichloride.
Structure
[edit]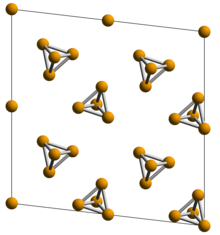
White phosphorus exists as molecules of four phosphorus atoms in a tetrahedral structure, joined by six phosphorus—phosphorus single bonds. The tetrahedral arrangement results in ring strain and instability.[3] Although both are called "white phosphorus", in fact two different crystal allotropes are known, interchanging reversibly at 195.2 K.[4] The element's standard state is the body-centered cubic α form, which is actually metastable under standard conditions.[3] The β form is believed to have a hexagonal crystal structure.[4]
Molten and gaseous white phosphorus also retains the tetrahedral molecules, until 800 °C (1,500 °F; 1,100 K) when it starts decomposing to P
2 molecules.[5] The P
4 molecule in the gas phase has a P-P bond length of rg = 2.1994(3) Å as was determined by gas electron diffraction.[6] The β form of white phosphorus contains three slightly different P
4 molecules, i.e. 18 different P-P bond lengths — between 2.1768(5) and 2.1920(5) Å. The average P-P bond length is 2.183(5) Å.[5]
Chemical properties
[edit]Despite white phosphorus not being the most stable allotropes of phosphorus, its molecular nature allows it to be easily purified. Thus, it's defined to have a zero enthalpy of formation.
In base, white phosphorus spontaneously disproportionates to phosphine and various phosphorus oxyacid salts.[7]
Many reactions of white phosphorus involve insertion into the P-P bonds, such as the reaction with oxygen, sulfur, phosphorus tribromide and the NO+ ion.
It ignites spontaneously in air at about 50 °C (122 °F), and at much lower temperatures if finely divided (due to melting-point depression). Phosphorus reacts with oxygen, usually forming two oxides depending on the amount of available oxygen: P4O6 (phosphorus trioxide) when reacted with a limited supply of oxygen, and P4O10 when reacted with excess oxygen. On rare occasions, P4O7, P4O8, and P4O9 are also formed, but in small amounts. This combustion gives phosphorus(V) oxide:
- P4 + 5 O2 → P4O10
Production and applications
[edit]The white allotrope can be produced using several methods. In the industrial process, phosphate rock is heated in an electric or fuel-fired furnace in the presence of carbon and silica.[8] Elemental phosphorus is then liberated as a vapour and can be collected under phosphoric acid. An idealized equation for this carbothermal reaction is shown for calcium phosphate (although phosphate rock contains substantial amounts of fluoroapatite, which would also form silicon tetrafluoride):
- 2 Ca3(PO4)2 + 6 SiO2 + 10 C → 6 CaSiO3 + 10 CO + P4
In this way, an estimated 750,000 tons were produced in 1988.[9]
Most (83% in 1988) white phosphorus is used as a precursor to phosphoric acid, half of which is used for food or medical products where purity is important. The other half is used for detergents. Much of the remaining 17% is mainly used for the production of chlorinated compounds phosphorus trichloride, phosphorus oxychloride, and phosphorus pentachloride:[10]
- P4 + 10Cl2 → 4PCl5
Other products derived from white phosphorus include phosphorus pentasulfide and various metal phosphides.[9]
Other polyhedrane analogues
[edit]Although white phosphorus forms the tetrahedron, the simplest possible Platonic hydrocarbon, no other polyhedral phosphorus clusters are known.[11] White phosphorus converts to the thermodynamically-stabler red allotrope, but that allotrope is not isolated polyhedra.
Cubane, in particular, is unlikely to form,[11] and the closest approach is the half-phosphorus compound P4(CH)4, produced from phosphaalkynes.[12] Other clusters are more thermodynamically favorable, and some have been partially formed as components of larger polyelemental compounds.[11]
Safety
[edit]White phosphorus is rather acutely toxic, with a lethal dose of 50-100 mg (1 mg/kg body weight). Its mode of action is thought to involve its reducing properties. It is metabolized to phosphate, which is not toxic.[9]
White phosphorus is used as a weapon because it is pyrophoric. For the same reasons, it is dangerous to handle. Measures are taken to protect samples from air. Anecdotal report of problems for beachcombers who may collect washed-up samples while unaware of their true nature.[13][14]
See also
[edit]References
[edit]- ^ "White phosphorus". American Chemical Society. Retrieved 2024-08-10.
- ^ Weeks, Mary Elvira (1932). "The discovery of the elements. II. Elements known to the alchemists". Journal of Chemical Education. 9 (1): 11. Bibcode:1932JChEd...9...11W. doi:10.1021/ed009p11.
- ^ a b Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. p. 392. ISBN 978-0-13-039913-7.
- ^ a b Durif, A.; Averbuch-Pouchot, M.T. (1996). Topics in phosphate chemistry. Singapore [u.a.]: World Scientific. p. 3. ISBN 978-981-02-2634-3.
- ^ a b Simon, Arndt; Borrmann, Horst; Horakh, Jörg (1997). "On the Polymorphism of White Phosphorus". Chemische Berichte. 130 (9): 1235–1240. doi:10.1002/cber.19971300911.
- ^ Cossairt, Brandi M.; Cummins, Christopher C.; Head, Ashley R.; Lichtenberger, Dennis L.; Berger, Raphael J. F.; Hayes, Stuart A.; Mitzel, Norbert W.; Wu, Gang (2010-06-01). "On the Molecular and Electronic Structures of AsP3 and P4". Journal of the American Chemical Society. 132 (24): 8459–8465. doi:10.1021/ja102580d. ISSN 0002-7863. PMID 20515032.
- ^ Engel, Robert; Cohen, JaimeLee Iolani (2004). Synthesis of Carbon-Phosphorus Bonds (2nd ed.). Boca Raton: CRC Press. §2.3. ISBN 0-8493-1617-0. LCCN 2003060796.
- ^ Threlfall, R.E., (1951). 100 years of Phosphorus Making: 1851–1951. Oldbury: Albright and Wilson Ltd
- ^ a b c Diskowski, Herbert; Hofmann, Thomas (2000). "Phosphorus". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a19_505. ISBN 978-3-527-30385-4.
- ^ Chemistry Part I Class XII (PDF) (Reprinted ed.). India: NCERT. January 2019. p. 177. ISBN 978-81-7450-648-1.
- ^ a b c Corbridge, D. E. C. (1995) "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam. § 4.1.12. ISBN 0-444-89307-5.
- ^ Streubel, Rainer (1995). "Phosphaalkyne Cyclooligomers: From Dimers to Hexamers—First Steps on the Way to Phosphorus–Carbon Cage Compounds". Angewandte Chemie International Edition in English. 34 (4): 436–438. doi:10.1002/anie.199504361.
- ^ "A dangerous guide to beachcombing".
- ^ "Woman mistakes WWII-era munition for precious stone on German beach | DW | 05.08.2017". Deutsche Welle.
