Amurensin (flavonol)

 | |
 | |
| Names | |
|---|---|
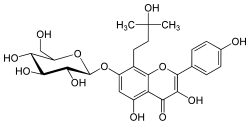
| IUPAC name 7-(β-D-Glucopyranosyloxy)-3,4′,5-trihydroxy-8-(3-hydroxy-3-methylbutyl)flavone | |
| Systematic IUPAC name 3,5-Dihydroxy-8-(3-hydroxy-3-methylbutyl)-2-(4-hydroxyphenyl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C26H30O12 | |
| Molar mass | 534.50 g/mol |
| Density | 1.581 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Amurensin is a flavonol, a type of flavonoid. It is the tert-amyl alcohol derivative of kaempferol 7-O-glucoside. It can be found in Phellodendron amurense.[1]
Related compounds
[edit]6"'-O-acetyl amurensin is found in the leaves of Phellodendron japonicum.[2]
References
[edit]- ^ Two New Flavonoid Glycosides from the Leaves of Phellodendron amurense Ruprecht. Masao Hasegawa and Teruo Shirato, J. Am. Chem. Soc., 1953, 75 (22), pages 5507–5511, doi:10.1021/ja01118a013
- ^ Constituents of Leaves of Phellodendron japonicum MAXIM. and Their Antioxidant Activity, Chih-Yang Chiu, Chia-Ying Li, Chao-Chen Chiu, Masatake Niwa, Susumu Kitanaka, Amooru Gangaiah Damu, E-Jian Lee and Tian-Shung Wu, Chem. Pharm. Bull., Vol. 53, pages 1118-1121 (2005), doi:10.1248/cpb.53.1118