Eupalitin

 | |
| Names | |
|---|---|
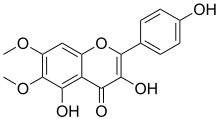
| IUPAC name 3,4′,5-Trihydroxy-6,7-dimethoxyflavone | |
| Systematic IUPAC name 3,5-Dihydroxy-2-(4-hydroxyphenyl)-6,7-dimethoxy-4H-1-benzopyran-4-one | |
| Other names Betuletol | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C17H14O7 | |
| Molar mass | 330.292 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Eupalitin is an O-methylated flavonol. It can be found in Ipomopsis aggregata.[1]
Glycosides
[edit]Eupalitin 3-O-β-D-galactopyranoside can be isolated from Tephrosia spinosa.[2]
Eupalin is the eupalitin 30-rhamnoside.
References
[edit]- ^ Identification of eupalitin, eupatolitin and patuletin glycosides in Ipomopsis aggregata. D.M. Smith, C.W. Glennie and J.B. Harborne, Phytochemistry, Volume 10, Issue 12, December 1971, pp. 3115-3120, doi:10.1016/S0031-9422(00)97361-8
- ^ Eupalitin 3-O-β-D-galactopyranoside from Tephrosia spinosa. Vanangamudi A., Gandhidasan R. and Raman P. V., Fitoterapia, 1997, vol. 68, no6, p. 560, INIST 2113413