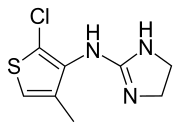
Tiamenidine
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
| Clinical data | |
|---|---|
| Trade names | Sundralen, Symcorad, Symcor |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 2.3–5 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C8H10ClN3S |
| Molar mass | 215.70 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tiamenidine (BAN, USAN, INN, also known as thiamenidine, Hoe 440) is an imidazoline compound that shares many of the pharmacological properties of clonidine. It is a centrally-acting α2 adrenergic receptor agonist (IC50 = 9.1 nM).[2] It also acts as an α1-adrenergic receptor agonist to a far lesser extent (IC50 = 4.85 μM).[2] In hypertensive volunteers, like clonidine, it significantly increased sinus node recovery time and lowered cardiac output.[3] It was marketed (as tiamenidine hydrochloride) by Sanofi-Aventis[4] under the brand name Sundralen[5] for the management of essential hypertension.[6]
Synthesis
[edit]
Reaction of thiourea 1 with methyl iodide gives the corresponding S-methyl analogue (2), followed by heating with ethylenediamine, completes the synthesis of tiamenidine (3).
See also
[edit]References
[edit]- ^ Eckert HG, Baudner S, Weimer KE, Wissmann H (1981). "Determination of tiamenidine in biological specimens by radioimmunoassay". Arzneimittel-Forschung. 31 (3): 419–24. PMID 7194666.
- ^ a b Timmermans PB, de Jonge A, Thoolen MJ, Wilffert B, Batink H, van Zwieten PA (April 1984). "Quantitative relationships between alpha-adrenergic activity and binding affinity of alpha-adrenoceptor agonists and antagonists". Journal of Medicinal Chemistry. 27 (4): 495–503. doi:10.1021/jm00370a011. PMID 6142954.
- ^ Roden DM, Nadeau JH, Primm RK (June 1988). "Electrophysiologic and hemodynamic effects of chronic oral therapy with the alpha 2-agonists clonidine and tiamenidine in hypertensive volunteers". Clinical Pharmacology and Therapeutics. 43 (6): 648–54. doi:10.1038/clpt.1988.90. PMID 2897889. S2CID 44263714.
- ^ "Pharmaceutical and healthcare online databases. Tiamenidine Hydrochloride". Drugs-About.com. Retrieved 30 November 2015.
- ^ Ganten D, Mulrow PJ, eds. (2013). Pharmacology of Antihypertensive Therapeutics (1st ed.). [S.l.]: Springer-Verlag Berlin Heidelberg. p. 880. ISBN 978-3-642-74211-8.
- ^ Zamboulis C, Hossmann V, Dollery CT, Eckert H (October 1979). "Tiamenidine, a centrally acting antihypertensive drug in essential hypertension [proceedings]". British Journal of Clinical Pharmacology. 8 (4): 390P. doi:10.1111/j.1365-2125.1979.tb04737.x. PMID 508528.
- ^ Castaer, J.; Thorpe, P.; Tiamenidine. Drugs Fut 1976, 1, 10, 502.
- ^ US 3758476, 0 Rippel H, Ruschig H, Linder E, Schorr M, issued 1973