Polyestriol phosphate

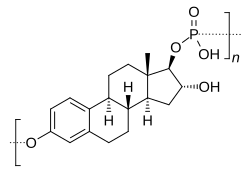 | |
 Skeletal structure of polyestriol phosphate (top) and ball-and-stick model of estriol phosphate (one monomer of polyestriol phosphate) (bottom) | |
| Clinical data | |
|---|---|
| Trade names | Gynäsan, Klimadurin, Triodurin |
| Other names | PE3P; SEP; Poly(estriol phosphate); Estriol phosphate polymer; Estriol polymer with phosphoric acid |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High |
| Metabolites | Estriol, phosphoric acid, and metabolites of estriol |
| Excretion | Urine (as conjugates) |
| Identifiers | |
| |
| CAS Number | |
| UNII | |
| Chemical and physical data | |
| Formula | (C18H23O5P)n (n = variable) |
| Molar mass | Polymer: Variable Repeat unit: 350.346 g/mol |
Polyestriol phosphate (PE3P, SEP), sold under the brand names Gynäsan, Klimadurin, and Triodurin, is an estrogen medication which was previously used in menopausal hormone therapy (i.e., to treat menopausal symptoms in postmenopausal women) and is no longer available.[1][2][3][4][5][6][7][8][9][10][11][12]
Medical uses
[edit]PE3P has been used at a dosage of 40 to 80 mg by intramuscular injection once every 4 to 8 weeks in menopausal hormone therapy.[13][10]
Available forms
[edit]PE3P has been available in the form of ampoules containing 50 to 80 mg in 1 or 2 mL aqueous solution.[13][14][15]
Pharmacology
[edit]PE3P is similar to polyestradiol phosphate (PEP), and is, likewise, an estrogen ester – specifically, an ester and prodrug of estriol – in the form of a polymer with phosphate linkers.[2][4][5][9] When adjusted for differences in molecular weight, PE3P contains the equivalent of about 80% of the amount of estriol.[16] As such, 40 mg PE3P corresponds to about 32 mg estriol.[16] Doses of PE3P of 10 mg or more have an extended duration of action.[17] A single intramuscular injection of 80 mg PE3P has a duration of about 1 month and of 80 mg about 2 months.[5][14]
The effects of PE3P on the vagina, uterus, pregnancy, prostate gland, coagulation, and fibrinolysis, as well as on mammary and endometrial cancer risk, have been studied.[18][19][20][21][22][23][24][25][16] The endometrial proliferation dose of PE3P over 14 days in women is 40 to 60 mg by intramuscular injection.[14]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Chemistry
[edit]PE3P is a water-soluble polymer of estriol with phosphoric acid.[17]
History
[edit]PE3P was developed by the Swedish pharmaceutical company Leo Läkemedel AB in the 1960s.[2][9][17] It was introduced for medical use by 1968.[26][27]
Society and culture
[edit]Brand names
[edit]PE3P was marketed under brand names including Gynäsan, Klimadurin, and Triodurin.[6][13][14][15][28]
Availability
[edit]PE3P was marketed in Germany and Spain.[14][29]
See also
[edit]References
[edit]- ^ Konyves I (January 1965). "En vattenloslig ostriolpolymer-polyostriolfosfat" [A water-soluble estriol polymer-polyestriol phosphate]. Tidsskrift for Kjemi Bergvesenog Metallurgi. 25 (12): 288.
- ^ a b c Lauritzen C, Velibese S (September 1961). "Clinical investigations of a long-acting oestriol (polyoestriol phosphate)". Acta Endocrinologica. 38 (1): 73–87. doi:10.1530/acta.0.0380073. PMID 13759555.
- ^ Lauritzen C (May 1968). "[Experience with an injectionable estriol Depot preparation. (Gynaesan pro injectione)]". Munchener Medizinische Wochenschrift (in German). 110 (19): 1203–1206. PMID 5695211.
- ^ a b Bachmann FF (January 1971). "[Treatment of menopausal complants with polyoestriol-phosphate. Experiences with Gynäsan injections]" [Treatment of menopausal complants with polyoestriol-phosphate. Experiences with Gynäsan injections]. Munchener Medizinische Wochenschrift (in German). 113 (5): 166–169. PMID 5107471.
- ^ a b c Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 551–. ISBN 978-3-642-96158-8.
The polymer of estradiol or estriol and phosphoric acid has an excellent depot action when given intramuscularly (polyestriol phosphate or polyestradiol phosphate) (Table 16). Phosphoric acid combines with the estrogen molecule at C3 and C17 to form a macromolecule. The compound is stored in the liver and spleen where the estrogen is steadily released by splitting off of the phosphate portion due to the action of alkaline phosphatase. [...] Conjugated estrogens and polyestriol and estradiol phosphate can also be given intravenously in an aqueous solution. Intravenous administration of ovarian hormones offers no advantages, however, and therefore has no practical significance. [...] The following duarations of action have been obtained with a single administration (WlED, 1954; LAURITZEN, 1968): [...] 50 mg polyestradiol phosphate ~ 1 month; 50 mg polyestriol phosphate ~ 1 month; 80 mg polyestriol phosphate ~ 2 months.
- ^ a b Negwer M, Scharnow HG (4 October 2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. ISBN 978-3-527-30247-5.
8075-01 (6628-01) 37452-43-0 R Polymeric ester with phosphoric acid S Klimadurin, Polyestriol phosphate, Polyostriolphosphat, Triodurin U Depot-estrogen
- ^ Martindale W, Royal Pharmaceutical Society of Great Britain. Dept. of Pharmaceutical Sciences (1993). The Extra Pharmacopoeia. Pharmaceutical Press. p. 2258. ISBN 978-0-85369-300-0.
Polyoestriol Phosphate. [...] ingredient of Klimadurin. [...] Triodurin [...].
- ^ Archiv für Gynäkologie. 1971. p. 206.
Polyoestriol phosphat. Gynäsan (R) pro injectione, 50 mg als Depot.
- ^ a b c Ars Medici. 1971. pp. 194–196, 408, 786.
Klinik in Lund die wirküng von Östriol an einem Urethritismaterial untersucht. Bei dem Präparat, Triodurin1-Leo, das bei der Prüfung verwendet wurde, handelt es sich um ein Polyöstriolphosphat, ein Polyester aus östra-1,3,5(10)-triene-3,16α,17β-triol und Phosphorsäure. Das östriolmolekül wurde mittels Phosphorsäurebrücken in die polymere Form gebracht, und das Molekül ent-hält eine grosse Zahl von Östrioleinheiten (Kön vves, 1965). Das Präparat besitzt [...] Behandlung und Ergebnisse Sämtliche Patientinnen erhielten etwa jeden 2. Monat 50-80 mg Poly-ostriolphosphat. Die Frauen reagierten fast ausnahmslos zufriedenstellend auf diese Behandlung. Die meisten gaben spontane Besserung ihrer übrigen klimak- [...] Eigenschaften Klimadurin F. Ist ein aus natürlichem Oestriol gewonnenes Depot-östrogen. Das wasserlösliche, aber inaktive polymerisierte östriol-phosphat wird durch langsamen Abbau als biologisch wirksame Form kontinuierlich freigesetzt. Die östrogene Wirkung äussert sich fast ausschliesslich in einer Stimullerung der Cervixdrüsen und der Proli- [...]
- ^ a b Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ^ Utian WH (6 December 2012). The Menopause Manual: A woman's guide to the menopause. Springer Science & Business Media. pp. 58–. ISBN 978-94-011-7135-9.
- ^ Campbell S (6 December 2012). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. Springer Science & Business Media. pp. 395–. ISBN 978-94-011-6165-7.
In the Federal Republic of Germany between 10 and 20% of all climacteric women are on estrogen treatment. We have the following oral estrogens for a treatment. (t) Conjugated estrogens, (2) estradiol valerate, (3) ethinyl-estradiol and its cyclopentyl-enol ether, (4) stilbestrol, (5) ethinyl-estradiol-methyltestosterone, (6) estriol and estriol succinate, most of them as coated tablets. Several long acting injectable preparations are available: several esters of combined estradiol-testosterone, one of estradiol-dehydroepiandrosterone enanthate and a prolonged polyestriol phosphate are also available. Lastly, depot injections of estradiol- and stilbestrol-esters are on the market.
- ^ a b c Saure A (11 November 2013). Die Wechseljahre der Frau: Hormone — Präparate — Therapien. Springer-Verlag. pp. 156–. ISBN 978-3-0348-6676-7.
Polyestriol phosphate 50 mg*** and 80 mg/1 ml*** and 2 ml solution for injection = 1 ampoule (Klimadurin*, Triodurin***). Recommended dosage: 1 ampoule in the muscle every 6–8 weeks.
- ^ a b c d e Lauritzen C (1988). "Natürliche und Synthetische Sexualhormone – Biologische Grundlagen und Behandlungsprinzipien" [Natural and Synthetic Sexual Hormones – Biological Basis and Medical Treatment Principles]. In Schneider HP, Lauritzen C, Nieschlag E (eds.). Grundlagen und Klinik der Menschlichen Fortpflanzung [Foundations and Clinic of Human Reproduction] (in German). Walter de Gruyter. pp. 229–306. ISBN 978-3110109689. OCLC 35483492.
- ^ a b Lauritzen C (23 January 1975). "Grundlagen der hormonalen Therapie klimakterischer Beschwerden" [Basics of Hormonal Therapy for Menopausal Symptoms] (PDF). Deutsches Ärzteblatt. 72 (4): 205–212. ISSN 0012-1207.
- ^ a b c Terenius L (February 1971). "Effect of anti-oestrogens on initiation of mammary cancer in the female rat". European Journal of Cancer. 7 (1): 65–70. doi:10.1016/0014-2964(71)90096-X. PMID 5576730.
- ^ a b c Johnson W (1962). "Therapeutic Trends". American Journal of Health-System Pharmacy. 19 (3): 136. doi:10.1093/ajhp/19.3.136. ISSN 1079-2082.
- ^ Sjöstedt S, Strandh J (1971). "Effect of polyestriol phosphate on the vaginal cytology and uterine endometrium of postmenopausal women". Acta Obstetricia et Gynecologica Scandinavica Supplement. 9 (Suppl 9): Suppl 9:30. doi:10.3109/00016347109161433. PMID 5287101. S2CID 73354582.
- ^ Purola E, Vartiainen E (1977). "Effect of long-acting oestriol on the vaginal cytology of postmenopausal women". Annales Chirurgiae et Gynaecologiae. 66 (4): 216–218. PMID 907314.
- ^ Fredholm B, Lindskog M (January 1969). "Some effects of a long-acting estriol polymer, polyestriol phosphate". Acta Physiologica Scandinavica: 74. Archived from the original on 2019-08-22. Retrieved 2018-11-11.
- ^ Müntzing J (1969). "Effects of polymerized oestrogens on the ventral prostate in rats". Acta Pharmacologica et Toxicologica. 27 (6): 417–423. doi:10.1111/j.1600-0773.1969.tb00488.x. PMID 5395730.
- ^ Lutwak-Mann C, Hay MF (1964). "Effect of certain water-soluble oestrogens on rabbit blastocysts". Journal of Endocrinology. 30 (4): ix–x. Archived from the original on 2019-08-22. Retrieved 2018-11-11.
- ^ Andersson M, Müntzing J (1971). "Effects of oestrogen on phosphatase activity in the ventral prostate of intact, castrated, and androgen-treated castrated, adult rats". Acta Pharmacologica et Toxicologica. 30 (3): 193–202. doi:10.1111/j.1600-0773.1971.tb00650.x. PMID 5171939.
- ^ Gjønnaess H, Munkeby I, Frølich W, Vennerød AM, Fagerhol MK (1978). "Effect of estrogen treatment on coagulation and fibrinolysis in postmenopausal women. With special reference to cold activation of factor VII". Gynecologic and Obstetric Investigation. 9 (2–3): 109–123. doi:10.1159/000300974. PMID 738649.
- ^ Lauritzen C, Meier F (1984). "Risks of endometrial and mammary cancer morbidity and mortality in long-term oestrogen treatment". The Climacteric: An Update. Springer. pp. 207–216. doi:10.1007/978-94-009-5608-7_20. ISBN 978-94-010-8973-9.
- ^ Svensk Farmaceutisk Tidskrift. Sveriges Apotekareförbunds. 1973. pp. 713, 728.
Ibland är de postmenopausala besvären så uttalade att östrogenbehandling kan anses befogad. Leo har för denna terapi introducerat Triovex (1960) och det långverkande polyöstriolfosfatet Triodurin (1968). [...] Med andra ord, i Triodurin är östriolmolekyler kopplade med fosforsyrabryggor till en polymer.» Vilka kliniska erfarenheter har man av Triodurin? »Erfarenheterna har visat att Triodurin är särskilt lämpligt vid östrogenbehandling när man vill [...] 1968 TRIODURIN Estriol som genom forsforsyrebryggor bringats i polymer form och därigenom ger en prolongerad effekt.
- ^ Arnold M (September 1969). "[Treatment of climacteric complannt with Klimadurin]" [Treatment of climacteric complaint with Klimadurin]. Praxis (in German). 58 (37): 1159–1162. PMID 5820318.
- ^ Revue Médicale de la Suisse Romande. H. Georg. 1972. p. 780.
- ^ Drugs Available Abroad. Gale Research. 1991. p. 176. ISBN 978-0-8103-7177-4.
790 * POLYESTRIOL PHOSPHATE Countries Where Available and Release Dates: Spain (1971). Brand Names and Manufacturers: • Klimadurin — Pharmacia Iberica (Spain) Drug Action: Estrogen. Indications/Usage: Menopausal symptoms; postmenopausal symptoms; posthysterectomy symptoms; estrogen replacement. How Supplied: 80 mg vials. Dosage: 80 mg every 4-8 weeks I.M. Precautions/Warnings: Biliary tract disease; endometriosis; uterine fibroma; hypercalcemia associated with tumors or metabolic bone disease; porphyria. Contraindications: Mammary tumors; estrogen-dependent tumors; history of cholestatic icterus; thrombophlebitis; thrombosis; thromboembolic disturbances; vaginal bleeding; pregnancy; lactation. Interactions: Oral anticoagulants; rifampin. [...] U.S. Treatments: Conjugated estrogens are commonly prescribed for menopausal symptoms in the U.S.