Osimertinib
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
 | |
| Clinical data | |
|---|---|
| Trade names | Tagrisso, others |
| Other names | AZD9291, mereletinib, osimertinib mesilate (JAN JP), osimertinib mesylate (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616005 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Probably high[4] |
| Metabolism | Oxidation (CYP3A) |
| Elimination half-life | 48 hours |
| Excretion | Feces (68%), urine (14%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
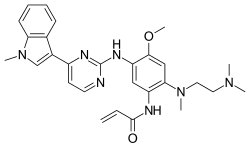
| Formula | C28H33N7O2 |
| Molar mass | 499.619 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Osimertinib, sold under the brand name Tagrisso,[6] is a medication used to treat non-small-cell lung carcinomas with specific mutations.[7][8] It is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor.
The most common side effects include diarrhea, rash, musculoskeletal pain, dry skin, skin inflammation around nails, sore mouth, fatigue and cough.[9]
Osimertinib was approved for medical use in the United States in November 2015,[10] and in the European Union in February 2016.[5]
Medical uses
[edit]Osimertinib is used to treat locally advanced or metastatic non-small-cell lung cancer (NSCLC), if the cancer cells are positive for the T790M mutation in the gene coding for EGFR or for activating EGFR mutations.[4][11] The T790M mutation may be de novo or acquired following first-line treatment with other EGFR tyrosine kinase inhibitors, such as gefitinib, erlotinib, and afatinib.[12]
In the US, EGFR exon 19 deletions, exon 21 L858R mutations or the T790M status of the patient prior to treatment with osimertinib must be detected by a companion diagnostic test.[4] The Food and Drug Administration (FDA) has approved multiple tests, including FoundationOne CDx for this purpose.[13] In Europe and elsewhere, activating EGFR mutations or T790M mutations may be determined by a validated test.[14]
In February 2024, the FDA approved osimertinib, in combination with platinum-based chemotherapy, for people with locally advanced or metastatic non-small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test.[15]
In people treated with osimertinib, resistance usually develops within approximately ten months.[16] Resistance mediated by an exon 20 C797S mutation accounts for the majority of resistance cases,[17] which has resulted in multiple attempts with non-ATP competitive or allosteric inhibitors to try and offset this acquired resistance by targeting other regions of the RTK kinase domain.[18]
In September 2024, the FDA approved osimertinib for adults with locally advanced, unresectable (stage III) non-small cell lung cancer (NSCLC) whose disease has not progressed during or following concurrent or sequential platinum-based chemoradiation therapy and whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test.[19]
Adverse effects
[edit]Very common (greater than 10% of clinical trial subjects) adverse effects include diarrhea, stomatitis, rashes, dry or itchy skin, infections where finger or toenails abut skin, low platelet counts, low leukocyte counts, and low neutrophil counts.[20]
Common (between 1% and 10% of clinical trial subjects) adverse effects include interstitial lung disease.[20]
It can cause fetal harm.[4][21]
Interactions
[edit]Osimertinib is metabolized by CYP3A4 and CYP3A5, so substances that strongly inhibit either enzyme, like macrolide antibiotics, antifungals, and antivirals may increase exposure to osimertinib, and substances like rifampicin that activate either enzyme may decrease the effectiveness of osimertinib.[4][20]
Pharmacology
[edit]Osimertinib preferentially binds irreversibly to mutated epidermal growth factor receptor proteins, particularly those with more common mutations in lung cancer such as L858R mutation or an exon 19 deletion.[4]
It exhibits linear pharmacokinetics; the median time to Cmax is 6 hours (range 3–24 hours). The estimated mean half-life is 48 hours, and oral clearance (CL/F) is 14.3 (L/h).[4] 68% of elimination is by feces and 14% by urine.[4]
Chemistry
[edit]Osimertinib is provided as the mesylate; the chemical formula is C28H33N7O2·CH4O3S, and the molecular weight is 596 g/mol. The chemical name is N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide mesylate salt.[4]
History
[edit]The drug discovery program that led to osimertinib started in 2009 and yielded the drug by 2012; the process was structure-driven and aimed to find a third generation EGFR inhibitor that would selectively target the T790M form of the EGFR receptor.[22]
Osimertinib was designated as a breakthrough therapy in April 2014, based on phase I trial results,[22] and the drug was provisionally approved under the FDA accelerated approval program with a priority review voucher, in November 2015.[23][10]
In February 2016, the EMA provisionally approved osimertinib under an accelerated process—the first approval under the program.[22][5]
In February 2024, the FDA approved osimertinib, in combination with platinum-based chemotherapy, for people with locally advanced or metastatic non-small cell lung cancer whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations, as detected by an FDA-approved test.[15] Efficacy was evaluated in FLAURA 2 (NCT04035486), an open-label, randomized trial of 557 participants with EGFR exon 19 deletion or exon 21 L858R mutation-positive la/mNSCLC and no prior systemic therapy for advanced disease.[15] Participants were randomized 1:1 to receive either osimertinib with platinum-based chemotherapy or osimertinib monotherapy.[15] The application was granted priority review, fast track, breakthrough therapy, and orphan drug designations.
Society and culture
[edit]Economics
[edit]At launch, in the United States, AstraZeneca priced the drug at US$12,750 per month.[24]: 59
References
[edit]- ^ "Prescription medicines: registration of new chemical entities in Australia, 2016". Therapeutic Goods Administration (TGA). 21 June 2022. Retrieved 10 April 2023.
- ^ "Product monograph brand safety updates". Health Canada. February 2024. Retrieved 24 March 2024.
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ a b c d e f g h i j "Tagrisso- osimertinib tablet, film coated". DailyMed. 5 June 2020. Retrieved 16 October 2020.
- ^ a b c "Tagrisso EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 16 October 2020.
- ^ "Proposed INN: List 113" (PDF). International Nonproprietary Names for Pharmaceutical Substances (INN). 29 (2): 285. 2015. Archived (PDF) from the original on 28 April 2017. Retrieved 16 November 2015.
- ^ Ayeni D, Politi K, Goldberg SB (September 2015). "Emerging Agents and New Mutations in EGFR-Mutant Lung Cancer". Clinical Cancer Research. 21 (17): 3818–20. doi:10.1158/1078-0432.CCR-15-1211. PMC 4720502. PMID 26169963.
- ^ Tan CS, Gilligan D, Pacey S (September 2015). "Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer". The Lancet. Oncology. 16 (9): e447–e459. doi:10.1016/S1470-2045(15)00246-6. PMID 26370354.
- ^ "FDA Approves First Adjuvant Therapy for Most Common Type of Lung Cancer". U.S. Food and Drug Administration (FDA) (Press release). 18 December 2020. Retrieved 20 December 2020.
- ^ a b "Tagrisso (osimertinib) tablets". U.S. Food and Drug Administration (FDA). 22 December 2015. Retrieved 16 October 2020.
- Lay summary in: "Summary review" (PDF). U.S. Food and Drug Administration (FDA). 10 November 2015.
- ^ Gijtenbeek RG, Damhuis RA, van der Wekken AJ, Hendriks LE, Groen HJ, van Geffen WH (April 2023). "Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in The Netherlands: a retrospective, nationwide registry study". The Lancet Regional Health. Europe. 27: 100592. doi:10.1016/j.lanepe.2023.100592. PMC 9932646. PMID 36817181.
- ^ Xu M, Xie Y, Ni S, Liu H (May 2015). "The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC)". Annals of Translational Medicine. 3 (7): 96. doi:10.3978/j.issn.2305-5839.2015.03.60. PMC 4430733. PMID 26015938.
- ^ Center for Devices and Radiological Health. "In Vitro Diagnostics - List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools)". www.fda.gov. Archived from the original on 25 January 2018. Retrieved 17 January 2018.
- ^ "European Tagrisso information" (PDF). European Medicines Agency. Archived (PDF) from the original on 17 January 2018. Retrieved 17 January 2018.
- ^ a b c d "FDA approves osimertinib with chemotherapy for EGFR-mutated non-small cell lung cancer". U.S. Food and Drug Administration. 16 February 2024. Retrieved 27 February 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ Patel H, Pawara R, Ansari A, Surana S (December 2017). "Recent updates on third generation EGFR inhibitors and emergence of fourth generation EGFR inhibitors to combat C797S resistance". European Journal of Medicinal Chemistry. 142: 32–47. doi:10.1016/j.ejmech.2017.05.027. PMID 28526474.
- ^ Wang S, Song Y, Liu D (January 2017). "EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance". Cancer Letters. 385: 51–54. doi:10.1016/j.canlet.2016.11.008. PMID 27840244.
- ^ Saraon P, Pathmanathan S, Snider J, Lyakisheva A, Wong V, Stagljar I (June 2021). "Receptor tyrosine kinases and cancer: oncogenic mechanisms and therapeutic approaches". Oncogene. 40 (24): 4079–4093. doi:10.1038/s41388-021-01841-2. PMID 34079087. S2CID 235307318.
- ^ "FDA approves osimertinib for locally advanced, unresectable (stage III) non-small cell lung cancer following chemoradiation therapy". U.S. Food and Drug Administration. 25 September 2024. Retrieved 27 September 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ^ a b c "UK label". UK Electronic Medicines Compendium. 26 January 2017. Archived from the original on 27 February 2017. Retrieved 27 February 2017.
- ^ Bollinger MK, Agnew AS, Mascara GP (July 2018). "Osimertinib: A third-generation tyrosine kinase inhibitor for treatment of epidermal growth factor receptor-mutated non-small cell lung cancer with the acquired Thr790Met mutation". Journal of Oncology Pharmacy Practice. 24 (5): 379–388. doi:10.1177/1078155217712401. PMID 28565936. S2CID 206671000.
- ^ a b c Yver A (June 2016). "Osimertinib (AZD9291)-a science-driven, collaborative approach to rapid drug design and development". Annals of Oncology. 27 (6): 1165–70. doi:10.1093/annonc/mdw129. PMID 26961148.
- ^ "Approved Drugs - Osimertinib". U.S. Food and Drug Administration (FDA). 13 November 2015. Archived from the original on 27 February 2017. Retrieved 27 February 2017.
- ^ "AHRQ Healthcare Horizon Scanning System – Potential High-Impact Interventions Report Priority Area 02: Cancer" (PDF). AHRQ. December 2015. Archived from the original (PDF) on 30 April 2017. Retrieved 27 February 2017.