Amphetamine
 From Wikipedia the free encyclopedia
From Wikipedia the free encyclopedia
Amphetamine[note 2] (contracted from alpha-methylphenethylamine) is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity; it is also used to treat binge eating disorder in the form of its inactive prodrug lisdexamfetamine. Amphetamine was discovered as a chemical in 1887 by Lazăr Edeleanu, and then as a drug in the late 1920s. It exists as two enantiomers:[note 3] levoamphetamine and dextroamphetamine. Amphetamine properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to the significant health risks associated with recreational use.[sources 1]
The first amphetamine pharmaceutical was Benzedrine, a brand which was used to treat a variety of conditions. Currently, pharmaceutical amphetamine is prescribed as racemic amphetamine, Adderall,[note 4] dextroamphetamine, or the inactive prodrug lisdexamfetamine. Amphetamine increases monoamine and excitatory neurotransmission in the brain, with its most pronounced effects targeting the norepinephrine and dopamine neurotransmitter systems.[sources 2]
At therapeutic doses, amphetamine causes emotional and cognitive effects such as euphoria, change in desire for sex, increased wakefulness, and improved cognitive control. It induces physical effects such as improved reaction time, fatigue resistance, decreased appetite, elevated heart rate, and increased muscle strength. Larger doses of amphetamine may impair cognitive function and induce rapid muscle breakdown. Addiction is a serious risk with heavy recreational amphetamine use, but is unlikely to occur from long-term medical use at therapeutic doses. Very high doses can result in psychosis (e.g., hallucinations, delusions and paranoia) which rarely occurs at therapeutic doses even during long-term use. Recreational doses are generally much larger than prescribed therapeutic doses and carry a far greater risk of serious side effects.[sources 3]
Amphetamine belongs to the phenethylamine class. It is also the parent compound of its own structural class, the substituted amphetamines,[note 5] which includes prominent substances such as bupropion, cathinone, MDMA, and methamphetamine. As a member of the phenethylamine class, amphetamine is also chemically related to the naturally occurring trace amine neuromodulators, specifically phenethylamine and N-methylphenethylamine, both of which are produced within the human body. Phenethylamine is the parent compound of amphetamine, while N-methylphenethylamine is a positional isomer of amphetamine that differs only in the placement of the methyl group.[sources 4]
Uses
Medical
Amphetamine is used to treat attention deficit hyperactivity disorder (ADHD), narcolepsy (a sleep disorder), obesity, and, in the form of lisdexamfetamine, binge eating disorder.[1][36][37] It is sometimes prescribed off-label for its past medical indications, particularly for depression and chronic pain.[1][52]
ADHD
Long-term amphetamine exposure at sufficiently high doses in some animal species is known to produce abnormal dopamine system development or nerve damage,[53][54] but, in humans with ADHD, long-term use of pharmaceutical amphetamines at therapeutic doses appears to improve brain development and nerve growth.[55][56][57] Reviews of magnetic resonance imaging (MRI) studies suggest that long-term treatment with amphetamine decreases abnormalities in brain structure and function found in subjects with ADHD, and improves function in several parts of the brain, such as the right caudate nucleus of the basal ganglia.[55][56][57]
Reviews of clinical stimulant research have established the safety and effectiveness of long-term continuous amphetamine use for the treatment of ADHD.[45][58][59] Randomized controlled trials of continuous stimulant therapy for the treatment of ADHD spanning 2 years have demonstrated treatment effectiveness and safety.[45][58] Two reviews have indicated that long-term continuous stimulant therapy for ADHD is effective for reducing the core symptoms of ADHD (i.e., hyperactivity, inattention, and impulsivity), enhancing quality of life and academic achievement, and producing improvements in a large number of functional outcomes[note 6] across 9 categories of outcomes related to academics, antisocial behavior, driving, non-medicinal drug use, obesity, occupation, self-esteem, service use (i.e., academic, occupational, health, financial, and legal services), and social function.[45][59] One review highlighted a nine-month randomized controlled trial of amphetamine treatment for ADHD in children that found an average increase of 4.5 IQ points, continued increases in attention, and continued decreases in disruptive behaviors and hyperactivity.[58] Another review indicated that, based upon the longest follow-up studies conducted to date, lifetime stimulant therapy that begins during childhood is continuously effective for controlling ADHD symptoms and reduces the risk of developing a substance use disorder as an adult.[45]
Current models of ADHD suggest that it is associated with functional impairments in some of the brain's neurotransmitter systems;[60] these functional impairments involve impaired dopamine neurotransmission in the mesocorticolimbic projection and norepinephrine neurotransmission in the noradrenergic projections from the locus coeruleus to the prefrontal cortex.[60] Stimulants like methylphenidate and amphetamine are effective in treating ADHD because they increase neurotransmitter activity in these systems.[27][60][61] Approximately 80% of those who use these stimulants see improvements in ADHD symptoms.[62] Children with ADHD who use stimulant medications generally have better relationships with peers and family members, perform better in school, are less distractible and impulsive, and have longer attention spans.[63][64] The Cochrane reviews[note 7] on the treatment of ADHD in children, adolescents, and adults with pharmaceutical amphetamines stated that short-term studies have demonstrated that these drugs decrease the severity of symptoms, but they have higher discontinuation rates than non-stimulant medications due to their adverse side effects.[66][67] A Cochrane review on the treatment of ADHD in children with tic disorders such as Tourette syndrome indicated that stimulants in general do not make tics worse, but high doses of dextroamphetamine could exacerbate tics in some individuals.[68]
Binge Eating Disorder
Binge eating disorder (BED) is characterized by recurrent and persistent episodes of compulsive binge eating.[69] These episodes are often accompanied by marked distress and a feeling of loss of control over eating.[69] The pathophysiology of BED is not fully understood, but it is believed to involve dysfunctional dopaminergic reward circuitry along the cortico-striatal-thalamic-cortical loop.[70][71] As of July 2024, lisdexamfetamine is the only USFDA- and TGA-approved pharmacotherapy for BED.[37][72] Evidence suggests that lisdexamfetamine's treatment efficacy in BED is underpinned at least in part by a psychopathological overlap between BED and ADHD, with the latter conceptualized as a cognitive control disorder that also benefits from treatment with lisdexamfetamine.[70][71]

Lisdexamfetamine's therapeutic effects for BED primarily involve direct action in the central nervous system after conversion to its pharmacologically active metabolite, dextroamphetamine.[72] Centrally, dextroamphetamine increases neurotransmitter activity of dopamine and norepinephrine in prefrontal cortical regions that regulate cognitive control of behavior.[70][72] Similar to its therapeutic effect in ADHD, dextroamphetamine enhances cognitive control and may reduce impulsivity in patients with BED by enhancing the cognitive processes responsible for overriding prepotent feeding responses that precede binge eating episodes.[70][74][75] In addition, dextroamphetamine's actions outside of the central nervous system may also contribute to its treatment effects in BED. Peripherally, dextroamphetamine triggers lipolysis through noradrenergic signaling in adipose fat cells, leading to the release of triglycerides into blood plasma to be utilized as a fuel substrate.[71][76] Dextroamphetamine also activates TAAR1 in peripheral organs along the gastrointestinal tract that are involved in the regulation of food intake and body weight.[73] Together, these actions confer an anorexigenic effect that promotes satiety in response to feeding and may decrease binge eating as a secondary effect.[75][73]
Medical reviews of randomized controlled trials have demonstrated that lisdexamfetamine, at doses between 50-70 mg, is safe and effective for the treatment of moderate-to-severe BED in adults.[sources 5] These reviews suggest that lisdexamfetamine is persistently effective at treating BED and is associated with significant reductions in the number of binge eating days and binge eating episodes per week.[sources 5] Furthermore, a meta-analytic systematic review highlighted an open-label, 12-month extension safety and tolerability study that reported lisdexamfetamine remained effective at reducing the number of binge eating days for the duration of the study.[75] In addition, both a review and a meta-analytic systematic review found lisdexamfetamine to be superior to placebo in several secondary outcome measures, including persistent binge eating cessation, reduction of obsessive-compulsive related binge eating symptoms, reduction of body-weight, and reduction of triglycerides.[71][75] Lisdexamfetamine, like all pharmaceutical amphetamines, has direct appetite suppressant effects that may be therapeutically useful in both BED and its comorbidities.[37][75] Based on reviews of neuroimaging studies involving BED-diagnosed participants, therapeautic neuroplasticity in dopaminergic and noradrenergic pathways from long-term use of lisdexamfetamine may be implicated in lasting improvements in the regulation of eating behaviors that are observed even after the drug is discontinued.[37][72][75]
Narcolepsy
Narcolepsy is a chronic sleep-wake disorder that is associated with excessive daytime sleepiness, cataplexy, and sleep paralysis.[78] Patients with narcolepsy are diagnosed as either type 1 or type 2, with only the former presenting cataplexy symptoms.[79] Type 1 narcolepsy results from the loss of approximately 70,000 orexin-releasing neurons in the lateral hypothalamus, leading to significantly reduced cerebrospinal orexin levels;[18][80] this reduction is a diagnostic biomarker for type 1 narcolepsy.[79] Lateral hypothalamic orexin neurons innervate every component of the ascending reticular activating system (ARAS), which includes noradrenergic, dopaminergic, histaminergic, and serotonergic nuclei that promote wakefulness.[80][81]
Amphetamine’s therapeutic mode of action in narcolepsy primarily involves increasing monoamine neurotransmitter activity in the ARAS.[18][82][83] This includes noradrenergic neurons in the locus coeruleus, dopaminergic neurons in the ventral tegmental area, histaminergic neurons in the tuberomammillary nucleus, and serotonergic neurons in the dorsal raphe nucleus.[81][83] Dextroamphetamine, the more dopaminergic enantiomer of amphetamine, is particularly effective at promoting wakefulness because dopamine release has the greatest influence on cortical activation and cognitive arousal, relative to other monoamines.[18] In contrast, levoamphetamine may have a greater effect on cataplexy, a symptom more sensitive to the effects of norepinephrine and serotonin.[18] Noradrenergic and serotonergic nuclei in the ARAS are involved in the regulation of the REM sleep cycle and function as "REM-off" cells, with amphetamine's effect on norepinephrine and serotonin contributing to the suppression of REM sleep and a possible reduction of cataplexy at high doses.[18][79][81]
The American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline conditionally recommends dextroamphetamine for the treatment of both type 1 and type 2 narcolepsy.[84] Treatment with pharmaceutical amphetamines is generally less preferred relative to other stimulants (e.g., modafinil) and is considered a third-line treatment option.[48][85][86] Medical reviews indicate that amphetamine is safe and effective for the treatment of narcolepsy.[18][48][84] Amphetamine appears to be most effective at improving symptoms associated with hypersomnolence, with three reviews finding clinically significant reductions in daytime sleepiness in patients with narcolepsy.[18][48][84] Additionally, these reviews suggest that amphetamine may dose-dependently improve cataplexy symptoms.[18][48][84] However, the quality of evidence for these findings is low and is consequently reflected in the AASM's conditional recommendation for dextroamphetamine as a treatment option for narcolepsy.[84]
Enhancing performance
Cognitive performance
In 2015, a systematic review and a meta-analysis of high quality clinical trials found that, when used at low (therapeutic) doses, amphetamine produces modest yet unambiguous improvements in cognition, including working memory, long-term episodic memory, inhibitory control, and some aspects of attention, in normal healthy adults;[87][88] these cognition-enhancing effects of amphetamine are known to be partially mediated through the indirect activation of both dopamine D1 receptor and α2-adrenergic receptor in the prefrontal cortex.[27][87] A systematic review from 2014 found that low doses of amphetamine also improve memory consolidation, in turn leading to improved recall of information.[89] Therapeutic doses of amphetamine also enhance cortical network efficiency, an effect which mediates improvements in working memory in all individuals.[27][90] Amphetamine and other ADHD stimulants also improve task saliency (motivation to perform a task) and increase arousal (wakefulness), in turn promoting goal-directed behavior.[27][91][92] Stimulants such as amphetamine can improve performance on difficult and boring tasks and are used by some students as a study and test-taking aid.[27][92][93] Based upon studies of self-reported illicit stimulant use, 5–35% of college students use diverted ADHD stimulants, which are primarily used for enhancement of academic performance rather than as recreational drugs.[94][95][96] However, high amphetamine doses that are above the therapeutic range can interfere with working memory and other aspects of cognitive control.[27][92]
Physical performance
Amphetamine is used by some athletes for its psychological and athletic performance-enhancing effects, such as increased endurance and alertness;[28][41] however, non-medical amphetamine use is prohibited at sporting events that are regulated by collegiate, national, and international anti-doping agencies.[97][98] In healthy people at oral therapeutic doses, amphetamine has been shown to increase muscle strength, acceleration, athletic performance in anaerobic conditions, and endurance (i.e., it delays the onset of fatigue), while improving reaction time.[28][99][100] Amphetamine improves endurance and reaction time primarily through reuptake inhibition and release of dopamine in the central nervous system.[99][100][101] Amphetamine and other dopaminergic drugs also increase power output at fixed levels of perceived exertion by overriding a "safety switch", allowing the core temperature limit to increase in order to access a reserve capacity that is normally off-limits.[100][102][103] At therapeutic doses, the adverse effects of amphetamine do not impede athletic performance;[28][99] however, at much higher doses, amphetamine can induce effects that severely impair performance, such as rapid muscle breakdown and elevated body temperature.[29][99]
Recreational
Amphetamine, specifically the more dopaminergic dextrorotatory enantiomer (dextroamphetamine), is also used recreationally as a euphoriant and aphrodisiac, and like other amphetamines; is used as a club drug for its energetic and euphoric high. Dextroamphetamine (d-amphetamine) is considered to have a high potential for misuse in a recreational manner since individuals typically report feeling euphoric, more alert, and more energetic after taking the drug.[104][105][106] A notable part of the 1960s mod subculture in the UK was recreational amphetamine use, which was used to fuel all-night dances at clubs like Manchester's Twisted Wheel. Newspaper reports described dancers emerging from clubs at 5 a.m. with dilated pupils.[107] Mods used the drug for stimulation and alertness, which they viewed as different from the intoxication caused by alcohol and other drugs.[107] Dr. Andrew Wilson argues that for a significant minority, "amphetamines symbolised the smart, on-the-ball, cool image" and that they sought "stimulation not intoxication [...] greater awareness, not escape" and "confidence and articulacy" rather than the "drunken rowdiness of previous generations."[107] Dextroamphetamine's dopaminergic (rewarding) properties affect the mesocorticolimbic circuit; a group of neural structures responsible for incentive salience (i.e., "wanting"; desire or craving for a reward and motivation), positive reinforcement and positively-valenced emotions, particularly ones involving pleasure.[108] Large recreational doses of dextroamphetamine may produce symptoms of dextroamphetamine overdose.[106] Recreational users sometimes open dexedrine capsules and crush the contents in order to insufflate (snort) it or subsequently dissolve it in water and inject it.[106] Immediate-release formulations have higher potential for abuse via insufflation (snorting) or intravenous injection due to a more favorable pharmacokinetic profile and easy crushability (especially tablets).[109][110] Injection into the bloodstream can be dangerous because insoluble fillers within the tablets can block small blood vessels.[106] Chronic overuse of dextroamphetamine can lead to severe drug dependence, resulting in withdrawal symptoms when drug use stops.[106]
Contraindications
According to the International Programme on Chemical Safety (IPCS) and the U.S. Food and Drug Administration (FDA),[note 8] amphetamine is contraindicated in people with a history of drug abuse,[note 9] cardiovascular disease, severe agitation, or severe anxiety.[36][29][112] It is also contraindicated in individuals with advanced arteriosclerosis (hardening of the arteries), glaucoma (increased eye pressure), hyperthyroidism (excessive production of thyroid hormone), or moderate to severe hypertension.[36][29][112] These agencies indicate that people who have experienced allergic reactions to other stimulants or who are taking monoamine oxidase inhibitors (MAOIs) should not take amphetamine,[36][29][112] although safe concurrent use of amphetamine and monoamine oxidase inhibitors has been documented.[113][114] These agencies also state that anyone with anorexia nervosa, bipolar disorder, depression, hypertension, liver or kidney problems, mania, psychosis, Raynaud's phenomenon, seizures, thyroid problems, tics, or Tourette syndrome should monitor their symptoms while taking amphetamine.[29][112] Evidence from human studies indicates that therapeutic amphetamine use does not cause developmental abnormalities in the fetus or newborns (i.e., it is not a human teratogen), but amphetamine abuse does pose risks to the fetus.[112] Amphetamine has also been shown to pass into breast milk, so the IPCS and the FDA advise mothers to avoid breastfeeding when using it.[29][112] Due to the potential for reversible growth impairments,[note 10] the FDA advises monitoring the height and weight of children and adolescents prescribed an amphetamine pharmaceutical.[29]
Adverse effects
The adverse side effects of amphetamine are many and varied, and the amount of amphetamine used is the primary factor in determining the likelihood and severity of adverse effects.[29][41] Amphetamine products such as Adderall, Dexedrine, and their generic equivalents are currently approved by the U.S. FDA for long-term therapeutic use.[38][29] Recreational use of amphetamine generally involves much larger doses, which have a greater risk of serious adverse drug effects than dosages used for therapeutic purposes.[41]
Physical
Cardiovascular side effects can include hypertension or hypotension from a vasovagal response, Raynaud's phenomenon (reduced blood flow to the hands and feet), and tachycardia (increased heart rate).[29][41][115] Sexual side effects in males may include erectile dysfunction, frequent erections, or prolonged erections.[29] Gastrointestinal side effects may include abdominal pain, constipation, diarrhea, and nausea.[1][29][116] Other potential physical side effects include appetite loss, blurred vision, dry mouth, excessive grinding of the teeth, nosebleed, profuse sweating, rhinitis medicamentosa (drug-induced nasal congestion), reduced seizure threshold, tics (a type of movement disorder), and weight loss.[sources 6] Dangerous physical side effects are rare at typical pharmaceutical doses.[41]
Amphetamine stimulates the medullary respiratory centers, producing faster and deeper breaths.[41] In a normal person at therapeutic doses, this effect is usually not noticeable, but when respiration is already compromised, it may be evident.[41] Amphetamine also induces contraction in the urinary bladder sphincter, the muscle which controls urination, which can result in difficulty urinating.[41] This effect can be useful in treating bed wetting and loss of bladder control.[41] The effects of amphetamine on the gastrointestinal tract are unpredictable.[41] If intestinal activity is high, amphetamine may reduce gastrointestinal motility (the rate at which content moves through the digestive system);[41] however, amphetamine may increase motility when the smooth muscle of the tract is relaxed.[41] Amphetamine also has a slight analgesic effect and can enhance the pain relieving effects of opioids.[1][41]
FDA-commissioned studies from 2011 indicate that in children, young adults, and adults there is no association between serious adverse cardiovascular events (sudden death, heart attack, and stroke) and the medical use of amphetamine or other ADHD stimulants.[sources 7] However, amphetamine pharmaceuticals are contraindicated in individuals with cardiovascular disease.[sources 8]
Psychological
At normal therapeutic doses, the most common psychological side effects of amphetamine include increased alertness, apprehension, concentration, initiative, self-confidence and sociability, mood swings (elated mood followed by mildly depressed mood), insomnia or wakefulness, and decreased sense of fatigue.[29][41] Less common side effects include anxiety, change in libido, grandiosity, irritability, repetitive or obsessive behaviors, and restlessness;[sources 9] these effects depend on the user's personality and current mental state.[41] Amphetamine psychosis (e.g., delusions and paranoia) can occur in heavy users.[29][42][43] Although very rare, this psychosis can also occur at therapeutic doses during long-term therapy.[29][43][44] According to the FDA, "there is no systematic evidence" that stimulants produce aggressive behavior or hostility.[29]
Amphetamine has also been shown to produce a conditioned place preference in humans taking therapeutic doses,[66][123] meaning that individuals acquire a preference for spending time in places where they have previously used amphetamine.[123][124]
Reinforcement disorders
Addiction
| Addiction and dependence glossary[124][125][126] | |
|---|---|
| |
| Transcription factor glossary | |
|---|---|
| |
Addiction is a serious risk with heavy recreational amphetamine use, but is unlikely to occur from long-term medical use at therapeutic doses;[46][47][48] in fact, lifetime stimulant therapy for ADHD that begins during childhood reduces the risk of developing substance use disorders as an adult.[45] Pathological overactivation of the mesolimbic pathway, a dopamine pathway that connects the ventral tegmental area to the nucleus accumbens, plays a central role in amphetamine addiction.[133][134] Individuals who frequently self-administer high doses of amphetamine have a high risk of developing an amphetamine addiction, since chronic use at high doses gradually increases the level of accumbal ΔFosB, a "molecular switch" and "master control protein" for addiction.[125][135][136] Once nucleus accumbens ΔFosB is sufficiently overexpressed, it begins to increase the severity of addictive behavior (i.e., compulsive drug-seeking) with further increases in its expression.[135][137] While there are currently no effective drugs for treating amphetamine addiction, regularly engaging in sustained aerobic exercise appears to reduce the risk of developing such an addiction.[138][139] Exercise therapy improves clinical treatment outcomes and may be used as an adjunct therapy with behavioral therapies for addiction.[138][140][sources 10]
Biomolecular mechanisms
Chronic use of amphetamine at excessive doses causes alterations in gene expression in the mesocorticolimbic projection, which arise through transcriptional and epigenetic mechanisms.[136][141][142] The most important transcription factors[note 11] that produce these alterations are Delta FBJ murine osteosarcoma viral oncogene homolog B (ΔFosB), cAMP response element binding protein (CREB), and nuclear factor-kappa B (NF-κB).[136] ΔFosB is the most significant biomolecular mechanism in addiction because ΔFosB overexpression (i.e., an abnormally high level of gene expression which produces a pronounced gene-related phenotype) in the D1-type medium spiny neurons in the nucleus accumbens is necessary and sufficient[note 12] for many of the neural adaptations and regulates multiple behavioral effects (e.g., reward sensitization and escalating drug self-administration) involved in addiction.[125][135][136] Once ΔFosB is sufficiently overexpressed, it induces an addictive state that becomes increasingly more severe with further increases in ΔFosB expression.[125][135] It has been implicated in addictions to alcohol, cannabinoids, cocaine, methylphenidate, nicotine, opioids, phencyclidine, propofol, and substituted amphetamines, among others.[sources 11]
ΔJunD, a transcription factor, and G9a, a histone methyltransferase enzyme, both oppose the function of ΔFosB and inhibit increases in its expression.[125][136][146] Sufficiently overexpressing ΔJunD in the nucleus accumbens with viral vectors can completely block many of the neural and behavioral alterations seen in chronic drug abuse (i.e., the alterations mediated by ΔFosB).[136] Similarly, accumbal G9a hyperexpression results in markedly increased histone 3 lysine residue 9 dimethylation (H3K9me2) and blocks the induction of ΔFosB-mediated neural and behavioral plasticity by chronic drug use,[sources 12] which occurs via H3K9me2-mediated repression of transcription factors for ΔFosB and H3K9me2-mediated repression of various ΔFosB transcriptional targets (e.g., CDK5).[136][146][147] ΔFosB also plays an important role in regulating behavioral responses to natural rewards, such as palatable food, sex, and exercise.[137][136][150] Since both natural rewards and addictive drugs induce the expression of ΔFosB (i.e., they cause the brain to produce more of it), chronic acquisition of these rewards can result in a similar pathological state of addiction.[137][136] Consequently, ΔFosB is the most significant factor involved in both amphetamine addiction and amphetamine-induced sexual addictions, which are compulsive sexual behaviors that result from excessive sexual activity and amphetamine use.[137][151][152] These sexual addictions are associated with a dopamine dysregulation syndrome which occurs in some patients taking dopaminergic drugs.[137][150]
The effects of amphetamine on gene regulation are both dose- and route-dependent.[142] Most of the research on gene regulation and addiction is based upon animal studies with intravenous amphetamine administration at very high doses.[142] The few studies that have used equivalent (weight-adjusted) human therapeutic doses and oral administration show that these changes, if they occur, are relatively minor.[142] This suggests that medical use of amphetamine does not significantly affect gene regulation.[142]
Pharmacological treatments
As of December 2019,[update] there is no effective pharmacotherapy for amphetamine addiction.[153][154][155] Reviews from 2015 and 2016 indicated that TAAR1-selective agonists have significant therapeutic potential as a treatment for psychostimulant addictions;[40][156] however, as of February 2016,[update] the only compounds which are known to function as TAAR1-selective agonists are experimental drugs.[40][156] Amphetamine addiction is largely mediated through increased activation of dopamine receptors and co-localized NMDA receptors[note 13] in the nucleus accumbens;[134] magnesium ions inhibit NMDA receptors by blocking the receptor calcium channel.[134][157] One review suggested that, based upon animal testing, pathological (addiction-inducing) psychostimulant use significantly reduces the level of intracellular magnesium throughout the brain.[134] Supplemental magnesium[note 14] treatment has been shown to reduce amphetamine self-administration (i.e., doses given to oneself) in humans, but it is not an effective monotherapy for amphetamine addiction.[134]
A systematic review and meta-analysis from 2019 assessed the efficacy of 17 different pharmacotherapies used in randomized controlled trials (RCTs) for amphetamine and methamphetamine addiction;[154] it found only low-strength evidence that methylphenidate might reduce amphetamine or methamphetamine self-administration.[154] There was low- to moderate-strength evidence of no benefit for most of the other medications used in RCTs, which included antidepressants (bupropion, mirtazapine, sertraline), antipsychotics (aripiprazole), anticonvulsants (topiramate, baclofen, gabapentin), naltrexone, varenicline, citicoline, ondansetron, prometa, riluzole, atomoxetine, dextroamphetamine, and modafinil.[154]
Behavioral treatments
A 2018 systematic review and network meta-analysis of 50 trials involving 12 different psychosocial interventions for amphetamine, methamphetamine, or cocaine addiction found that combination therapy with both contingency management and community reinforcement approach had the highest efficacy (i.e., abstinence rate) and acceptability (i.e., lowest dropout rate).[158] Other treatment modalities examined in the analysis included monotherapy with contingency management or community reinforcement approach, cognitive behavioral therapy, 12-step programs, non-contingent reward-based therapies, psychodynamic therapy, and other combination therapies involving these.[158]
Additionally, research on the neurobiological effects of physical exercise suggests that daily aerobic exercise, especially endurance exercise (e.g., marathon running), prevents the development of drug addiction and is an effective adjunct therapy (i.e., a supplemental treatment) for amphetamine addiction.[sources 10] Exercise leads to better treatment outcomes when used as an adjunct treatment, particularly for psychostimulant addictions.[138][140][159] In particular, aerobic exercise decreases psychostimulant self-administration, reduces the reinstatement (i.e., relapse) of drug-seeking, and induces increased dopamine receptor D2 (DRD2) density in the striatum.[137][159] This is the opposite of pathological stimulant use, which induces decreased striatal DRD2 density.[137] One review noted that exercise may also prevent the development of a drug addiction by altering ΔFosB or c-Fos immunoreactivity in the striatum or other parts of the reward system.[139]
| Form of neuroplasticity or behavioral plasticity | Type of reinforcer | Sources | |||||
|---|---|---|---|---|---|---|---|
| Opiates | Psychostimulants | High fat or sugar food | Sexual intercourse | Physical exercise (aerobic) | Environmental enrichment | ||
| ΔFosB expression in nucleus accumbens D1-type MSNs | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | [137] |
| Behavioral plasticity | |||||||
| Escalation of intake | Yes | Yes | Yes | [137] | |||
| Psychostimulant cross-sensitization | Yes | Not applicable | Yes | Yes | Attenuated | Attenuated | [137] |
| Psychostimulant self-administration | ↑ | ↑ | ↓ | ↓ | ↓ | [137] | |
| Psychostimulant conditioned place preference | ↑ | ↑ | ↓ | ↑ | ↓ | ↑ | [137] |
| Reinstatement of drug-seeking behavior | ↑ | ↑ | ↓ | ↓ | [137] | ||
| Neurochemical plasticity | |||||||
| CREB phosphorylation in the nucleus accumbens | ↓ | ↓ | ↓ | ↓ | ↓ | [137] | |
| Sensitized dopamine response in the nucleus accumbens | No | Yes | No | Yes | [137] | ||
| Altered striatal dopamine signaling | ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD1, ↓DRD2, ↑DRD3 | ↑DRD2 | ↑DRD2 | [137] | |
| Altered striatal opioid signaling | No change or ↑μ-opioid receptors | ↑μ-opioid receptors ↑κ-opioid receptors | ↑μ-opioid receptors | ↑μ-opioid receptors | No change | No change | [137] |
| Changes in striatal opioid peptides | ↑dynorphin No change: enkephalin | ↑dynorphin | ↓enkephalin | ↑dynorphin | ↑dynorphin | [137] | |
| Mesocorticolimbic synaptic plasticity | |||||||
| Number of dendrites in the nucleus accumbens | ↓ | ↑ | ↑ | [137] | |||
| Dendritic spine density in the nucleus accumbens | ↓ | ↑ | ↑ | [137] | |||
Dependence and withdrawal
Drug tolerance develops rapidly in amphetamine abuse (i.e., recreational amphetamine use), so periods of extended abuse require increasingly larger doses of the drug in order to achieve the same effect.[160][161] According to a Cochrane review on withdrawal in individuals who compulsively use amphetamine and methamphetamine, "when chronic heavy users abruptly discontinue amphetamine use, many report a time-limited withdrawal syndrome that occurs within 24 hours of their last dose."[162] This review noted that withdrawal symptoms in chronic, high-dose users are frequent, occurring in roughly 88% of cases, and persist for 3–4 weeks with a marked "crash" phase occurring during the first week.[162] Amphetamine withdrawal symptoms can include anxiety, drug craving, depressed mood, fatigue, increased appetite, increased movement or decreased movement, lack of motivation, sleeplessness or sleepiness, and lucid dreams.[162] The review indicated that the severity of withdrawal symptoms is positively correlated with the age of the individual and the extent of their dependence.[162] Mild withdrawal symptoms from the discontinuation of amphetamine treatment at therapeutic doses can be avoided by tapering the dose.[1]
Overdose
An amphetamine overdose can lead to many different symptoms, but is rarely fatal with appropriate care.[1][112][163] The severity of overdose symptoms increases with dosage and decreases with drug tolerance to amphetamine.[41][112] Tolerant individuals have been known to take as much as 5 grams of amphetamine in a day, which is roughly 100 times the maximum daily therapeutic dose.[112] Symptoms of a moderate and extremely large overdose are listed below; fatal amphetamine poisoning usually also involves convulsions and coma.[29][41] In 2013, overdose on amphetamine, methamphetamine, and other compounds implicated in an "amphetamine use disorder" resulted in an estimated 3,788 deaths worldwide (3,425–4,145 deaths, 95% confidence).[note 15][164]
| System | Minor or moderate overdose[29][41][112] | Severe overdose[sources 13] |
|---|---|---|
| Cardiovascular |
| |
| Central nervous system |
|
|
| Musculoskeletal |
| |
| Respiratory |
|
|
| Urinary |
| |
| Other |
|
|
Toxicity
In rodents and primates, sufficiently high doses of amphetamine cause dopaminergic neurotoxicity, or damage to dopamine neurons, which is characterized by dopamine terminal degeneration and reduced transporter and receptor function.[166][167] There is no evidence that amphetamine is directly neurotoxic in humans.[168][169] However, large doses of amphetamine may indirectly cause dopaminergic neurotoxicity as a result of hyperpyrexia, the excessive formation of reactive oxygen species, and increased autoxidation of dopamine.[sources 14] Animal models of neurotoxicity from high-dose amphetamine exposure indicate that the occurrence of hyperpyrexia (i.e., core body temperature ≥ 40 °C) is necessary for the development of amphetamine-induced neurotoxicity.[167] Prolonged elevations of brain temperature above 40 °C likely promote the development of amphetamine-induced neurotoxicity in laboratory animals by facilitating the production of reactive oxygen species, disrupting cellular protein function, and transiently increasing blood–brain barrier permeability.[167]
Psychosis
An amphetamine overdose can result in a stimulant psychosis that may involve a variety of symptoms, such as delusions and paranoia.[42][43] A Cochrane review on treatment for amphetamine, dextroamphetamine, and methamphetamine psychosis states that about 5–15% of users fail to recover completely.[42][172] According to the same review, there is at least one trial that shows antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[42] Psychosis rarely arises from therapeutic use.[29][43][44]
Drug interactions
Many types of substances are known to interact with amphetamine, resulting in altered drug action or metabolism of amphetamine, the interacting substance, or both.[29] Inhibitors of enzymes that metabolize amphetamine (e.g., CYP2D6 and FMO3) will prolong its elimination half-life, meaning that its effects will last longer.[6][29] Amphetamine also interacts with MAOIs, particularly monoamine oxidase A inhibitors, since both MAOIs and amphetamine increase plasma catecholamines (i.e., norepinephrine and dopamine);[29] therefore, concurrent use of both is dangerous.[29] Amphetamine modulates the activity of most psychoactive drugs. In particular, amphetamine may decrease the effects of sedatives and depressants and increase the effects of stimulants and antidepressants.[29] Amphetamine may also decrease the effects of antihypertensives and antipsychotics due to its effects on blood pressure and dopamine respectively.[29] Zinc supplementation may reduce the minimum effective dose of amphetamine when it is used for the treatment of ADHD.[note 16][177]
In general, there is no significant interaction when consuming amphetamine with food, but the pH of gastrointestinal content and urine affects the absorption and excretion of amphetamine, respectively.[29] Acidic substances reduce the absorption of amphetamine and increase urinary excretion, and alkaline substances do the opposite.[29] Due to the effect pH has on absorption, amphetamine also interacts with gastric acid reducers such as proton pump inhibitors and H2 antihistamines, which increase gastrointestinal pH (i.e., make it less acidic).[29]
Pharmacology
Pharmacodynamics
Pharmacodynamics of amphetamine in a dopamine neuron |
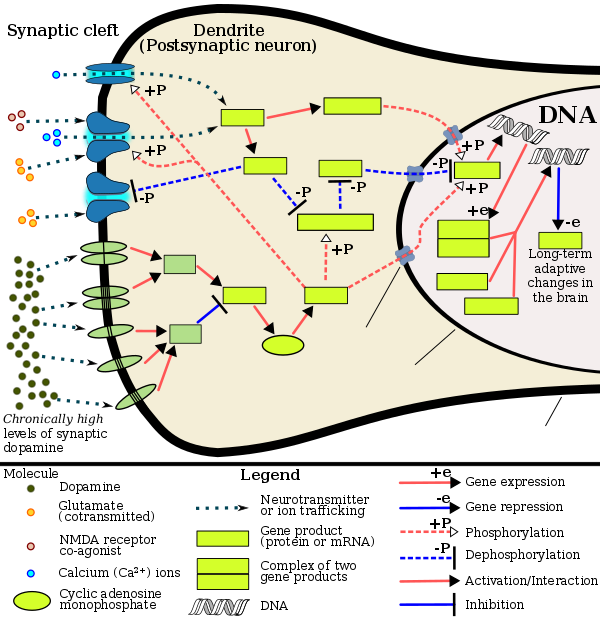
Amphetamine exerts its behavioral effects by altering the use of monoamines as neuronal signals in the brain, primarily in catecholamine neurons in the reward and executive function pathways of the brain.[39][61] The concentrations of the main neurotransmitters involved in reward circuitry and executive functioning, dopamine and norepinephrine, increase dramatically in a dose-dependent manner by amphetamine because of its effects on monoamine transporters.[39][61][178] The reinforcing and motivational salience-promoting effects of amphetamine are due mostly to enhanced dopaminergic activity in the mesolimbic pathway.[27] The euphoric and locomotor-stimulating effects of amphetamine are dependent upon the magnitude and speed by which it increases synaptic dopamine and norepinephrine concentrations in the striatum.[2]
Amphetamine has been identified as a potent full agonist of trace amine-associated receptor 1 (TAAR1), a Gs-coupled and Gq-coupled G protein-coupled receptor (GPCR) discovered in 2001, which is important for regulation of brain monoamines.[39][184] Activation of TAAR1 increases cAMP production via adenylyl cyclase activation and inhibits monoamine transporter function.[39][185] Monoamine autoreceptors (e.g., D2 short, presynaptic α2, and presynaptic 5-HT1A) have the opposite effect of TAAR1, and together these receptors provide a regulatory system for monoamines.[39][40] Notably, amphetamine and trace amines possess high binding affinities for TAAR1, but not for monoamine autoreceptors.[39][40] Imaging studies indicate that monoamine reuptake inhibition by amphetamine and trace amines is site specific and depends upon the presence of TAAR1 co-localization in the associated monoamine neurons.[39]
In addition to the neuronal monoamine transporters, amphetamine also inhibits both vesicular monoamine transporters, VMAT1 and VMAT2, as well as SLC1A1, SLC22A3, and SLC22A5.[sources 15] SLC1A1 is excitatory amino acid transporter 3 (EAAT3), a glutamate transporter located in neurons, SLC22A3 is an extraneuronal monoamine transporter that is present in astrocytes, and SLC22A5 is a high-affinity carnitine transporter.[sources 15] Amphetamine is known to strongly induce cocaine- and amphetamine-regulated transcript (CART) gene expression,[11][191] a neuropeptide involved in feeding behavior, stress, and reward, which induces observable increases in neuronal development and survival in vitro.[11][192][193] The CART receptor has yet to be identified, but there is significant evidence that CART binds to a unique Gi/Go-coupled GPCR.[193][194] Amphetamine also inhibits monoamine oxidases at very high doses, resulting in less monoamine and trace amine metabolism and consequently higher concentrations of synaptic monoamines.[23][195] In humans, the only post-synaptic receptor at which amphetamine is known to bind is the 5-HT1A receptor, where it acts as an agonist with low micromolar affinity.[196][197]
The full profile of amphetamine's short-term drug effects in humans is mostly derived through increased cellular communication or neurotransmission of dopamine,[39] serotonin,[39] norepinephrine,[39] epinephrine,[178] histamine,[178] CART peptides,[11][191] endogenous opioids,[198][199][200] adrenocorticotropic hormone,[201][202] corticosteroids,[201][202] and glutamate,[182][187] which it affects through interactions with CART, 5-HT1A, EAAT3, TAAR1, VMAT1, VMAT2, and possibly other biological targets.[sources 16] Amphetamine also activates seven human carbonic anhydrase enzymes, several of which are expressed in the human brain.[203]
Dextroamphetamine is a more potent agonist of TAAR1 than levoamphetamine.[204] Consequently, dextroamphetamine produces greater CNS stimulation than levoamphetamine, roughly three to four times more, but levoamphetamine has slightly stronger cardiovascular and peripheral effects.[41][204]
Dopamine
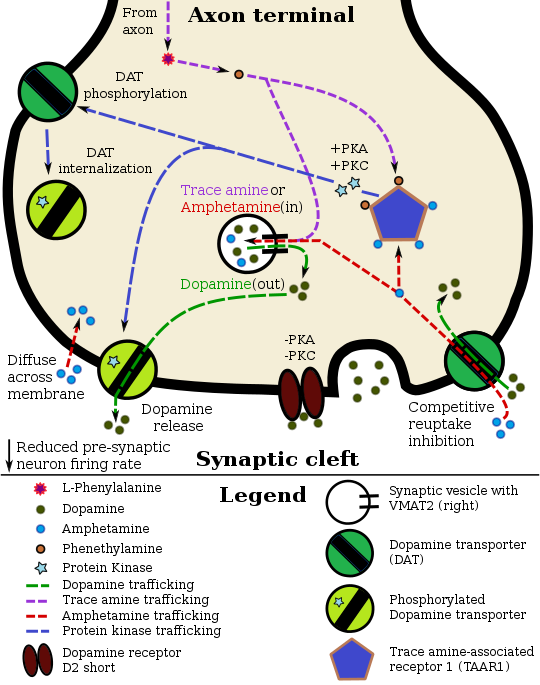
In certain brain regions, amphetamine increases the concentration of dopamine in the synaptic cleft.[39] Amphetamine can enter the presynaptic neuron either through DAT or by diffusing across the neuronal membrane directly.[39] As a consequence of DAT uptake, amphetamine produces competitive reuptake inhibition at the transporter.[39] Upon entering the presynaptic neuron, amphetamine activates TAAR1 which, through protein kinase A (PKA) and protein kinase C (PKC) signaling, causes DAT phosphorylation.[39] Phosphorylation by either protein kinase can result in DAT internalization (non-competitive reuptake inhibition), but PKC-mediated phosphorylation alone induces the reversal of dopamine transport through DAT (i.e., dopamine efflux).[note 16][39][205] Amphetamine is also known to increase intracellular calcium, an effect which is associated with DAT phosphorylation through an unidentified Ca2+/calmodulin-dependent protein kinase (CAMK)-dependent pathway, in turn producing dopamine efflux.[184][182][183] Through direct activation of G protein-coupled inwardly-rectifying potassium channels, TAAR1 reduces the firing rate of dopamine neurons, preventing a hyper-dopaminergic state.[180][181][206]
Amphetamine is also a substrate for the presynaptic vesicular monoamine transporter, VMAT2.[178][179] Following amphetamine uptake at VMAT2, amphetamine induces the collapse of the vesicular pH gradient, which results in the release of dopamine molecules from synaptic vesicles into the cytosol via dopamine efflux through VMAT2.[178][179] Subsequently, the cytosolic dopamine molecules are released from the presynaptic neuron into the synaptic cleft via reverse transport at DAT.[39][178][179]
Norepinephrine
Similar to dopamine, amphetamine dose-dependently increases the level of synaptic norepinephrine, the direct precursor of epinephrine.[49][61] Based upon neuronal TAAR1 mRNA expression, amphetamine is thought to affect norepinephrine analogously to dopamine.[39][178][205] In other words, amphetamine induces TAAR1-mediated efflux and non-competitive reuptake inhibition at phosphorylated NET, competitive NET reuptake inhibition, and norepinephrine release from VMAT2.[39][178]
Serotonin
Amphetamine exerts analogous, yet less pronounced, effects on serotonin as on dopamine and norepinephrine.[39][61] Amphetamine affects serotonin via VMAT2 and, like norepinephrine, is thought to phosphorylate SERT via TAAR1.[39][178] Like dopamine, amphetamine has low, micromolar affinity at the human 5-HT1A receptor.[196][197]
Other neurotransmitters, peptides, hormones, and enzymes
| Enzyme | KA (nM) | Sources |
|---|---|---|
| hCA4 | 94 | [203] |
| hCA5A | 810 | [203][207] |
| hCA5B | 2560 | [203] |
| hCA7 | 910 | [203][207] |
| hCA12 | 640 | [203] |
| hCA13 | 24100 | [203] |
| hCA14 | 9150 | [203] |
Acute amphetamine administration in humans increases endogenous opioid release in several brain structures in the reward system.[198][199][200] Extracellular levels of glutamate, the primary excitatory neurotransmitter in the brain, have been shown to increase in the striatum following exposure to amphetamine.[182] This increase in extracellular glutamate presumably occurs via the amphetamine-induced internalization of EAAT3, a glutamate reuptake transporter, in dopamine neurons.[182][187] Amphetamine also induces the selective release of histamine from mast cells and efflux from histaminergic neurons through VMAT2.[178] Acute amphetamine administration can also increase adrenocorticotropic hormone and corticosteroid levels in blood plasma by stimulating the hypothalamic–pituitary–adrenal axis.[36][201][202]
In December 2017, the first study assessing the interaction between amphetamine and human carbonic anhydrase enzymes was published;[203] of the eleven carbonic anhydrase enzymes it examined, it found that amphetamine potently activates seven, four of which are highly expressed in the human brain, with low nanomolar through low micromolar activating effects.[203] Based upon preclinical research, cerebral carbonic anhydrase activation has cognition-enhancing effects;[208] but, based upon the clinical use of carbonic anhydrase inhibitors, carbonic anhydrase activation in other tissues may be associated with adverse effects, such as ocular activation exacerbating glaucoma.[208]
Pharmacokinetics
The oral bioavailability of amphetamine varies with gastrointestinal pH;[29] it is well absorbed from the gut, and bioavailability is typically 90%.[10] Amphetamine is a weak base with a pKa of 9.9;[3] consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium.[3][29] Conversely, an acidic pH means the drug is predominantly in a water-soluble cationic (salt) form, and less is absorbed.[3] Approximately 20% of amphetamine circulating in the bloodstream is bound to plasma proteins.[11] Following absorption, amphetamine readily distributes into most tissues in the body, with high concentrations occurring in cerebrospinal fluid and brain tissue.[17]
The half-lives of amphetamine enantiomers differ and vary with urine pH.[3] At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively.[3] Highly acidic urine will reduce the enantiomer half-lives to 7 hours;[17] highly alkaline urine will increase the half-lives up to 34 hours.[17] The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively.[3] Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH.[3] When the urinary pH is basic, amphetamine is in its free base form, so less is excreted.[3] When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively.[3] Following oral administration, amphetamine appears in urine within 3 hours.[17] Roughly 90% of ingested amphetamine is eliminated 3 days after the last oral dose.[17]
Lisdexamfetamine is a prodrug of dextroamphetamine.[209][210] It is not as sensitive to pH as amphetamine when being absorbed in the gastrointestinal tract.[210] Following absorption into the blood stream, lisdexamfetamine is completely converted by red blood cells to dextroamphetamine and the amino acid L-lysine by hydrolysis via undetermined aminopeptidase enzymes.[210][209][211] This is the rate-limiting step in the bioactivation of lisdexamfetamine.[209] The elimination half-life of lisdexamfetamine is generally less than 1 hour.[210][209] Due to the necessary conversion of lisdexamfetamine into dextroamphetamine, levels of dextroamphetamine with lisdexamfetamine peak about one hour later than with an equivalent dose of immediate-release dextroamphetamine.[209][211] Presumably due to its rate-limited activation by red blood cells, intravenous administration of lisdexamfetamine shows greatly delayed time to peak and reduced peak levels compared to intravenous administration of an equivalent dose of dextroamphetamine.[209] The pharmacokinetics of lisdexamfetamine are similar regardless of whether it is administered orally, intranasally, or intravenously.[209][211] Hence, in contrast to dextroamphetamine, parenteral use does not enhance the subjective effects of lisdexamfetamine.[209][211] Because of its behavior as a prodrug and its pharmacokinetic differences, lisdexamfetamine has a longer duration of therapeutic effect than immediate-release dextroamphetamine and shows reduced misuse potential.[209][211]
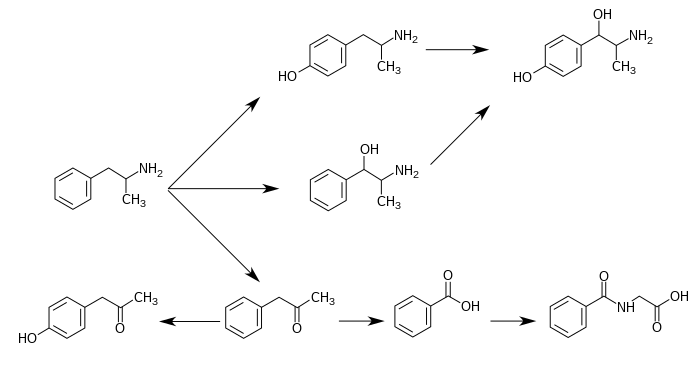
CYP2D6, dopamine β-hydroxylase (DBH), flavin-containing monooxygenase 3 (FMO3), butyrate-CoA ligase (XM-ligase), and glycine N-acyltransferase (GLYAT) are the enzymes known to metabolize amphetamine or its metabolites in humans.[sources 17] Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone.[3][12] Among these metabolites, the active sympathomimetics are 4-hydroxyamphetamine,[212] 4-hydroxynorephedrine,[213] and norephedrine.[214] The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination.[3][215] The known metabolic pathways, detectable metabolites, and metabolizing enzymes in humans include the following:
Metabolic pathways of amphetamine in humans[sources 17] |
Pharmacomicrobiomics
The human metagenome (i.e., the genetic composition of an individual and all microorganisms that reside on or within the individual's body) varies considerably between individuals.[221][222] Since the total number of microbial and viral cells in the human body (over 100 trillion) greatly outnumbers human cells (tens of trillions),[note 18][221][223] there is considerable potential for interactions between drugs and an individual's microbiome, including: drugs altering the composition of the human microbiome, drug metabolism by microbial enzymes modifying the drug's pharmacokinetic profile, and microbial drug metabolism affecting a drug's clinical efficacy and toxicity profile.[221][222][224] The field that studies these interactions is known as pharmacomicrobiomics.[221]
Similar to most biomolecules and other orally administered xenobiotics (i.e., drugs), amphetamine is predicted to undergo promiscuous metabolism by human gastrointestinal microbiota (primarily bacteria) prior to absorption into the blood stream.[224] The first amphetamine-metabolizing microbial enzyme, tyramine oxidase from a strain of E. coli commonly found in the human gut, was identified in 2019.[224] This enzyme was found to metabolize amphetamine, tyramine, and phenethylamine with roughly the same binding affinity for all three compounds.[224]
Related endogenous compounds
Amphetamine has a very similar structure and function to the endogenous trace amines, which are naturally occurring neuromodulator molecules produced in the human body and brain.[39][49][225] Among this group, the most closely related compounds are phenethylamine, the parent compound of amphetamine, and N-methylphenethylamine, a structural isomer of amphetamine (i.e., it has an identical molecular formula).[39][49][226] In humans, phenethylamine is produced directly from L-phenylalanine by the aromatic amino acid decarboxylase (AADC) enzyme, which converts L-DOPA into dopamine as well.[49][226] In turn, N-methylphenethylamine is metabolized from phenethylamine by phenylethanolamine N-methyltransferase, the same enzyme that metabolizes norepinephrine into epinephrine.[49][226] Like amphetamine, both phenethylamine and N-methylphenethylamine regulate monoamine neurotransmission via TAAR1;[39][225][226] unlike amphetamine, both of these substances are broken down by monoamine oxidase B, and therefore have a shorter half-life than amphetamine.[49][226]
Chemistry
Racemic amphetamine |
Phenyl-2-nitropropene (right cups)
Amphetamine is a methyl homolog of the mammalian neurotransmitter phenethylamine with the chemical formula C9H13N. The carbon atom adjacent to the primary amine is a stereogenic center, and amphetamine is composed of a racemic 1:1 mixture of two enantiomers.[11] This racemic mixture can be separated into its optical isomers:[note 19] levoamphetamine and dextroamphetamine.[11] At room temperature, the pure free base of amphetamine is a mobile, colorless, and volatile liquid with a characteristically strong amine odor, and acrid, burning taste.[22] Frequently prepared solid salts of amphetamine include amphetamine adipate,[227] aspartate,[29] hydrochloride,[228] phosphate,[229] saccharate,[29] sulfate,[29] and tannate.[230] Dextroamphetamine sulfate is the most common enantiopure salt.[50] Amphetamine is also the parent compound of its own structural class, which includes a number of psychoactive derivatives.[4][11] In organic chemistry, amphetamine is an excellent chiral ligand for the stereoselective synthesis of 1,1'-bi-2-naphthol.[231]
Substituted derivatives
The substituted derivatives of amphetamine, or "substituted amphetamines", are a broad range of chemicals that contain amphetamine as a "backbone";[4][51][232] specifically, this chemical class includes derivative compounds that are formed by replacing one or more hydrogen atoms in the amphetamine core structure with substituents.[4][51][233] The class includes amphetamine itself, stimulants like methamphetamine, serotonergic empathogens like MDMA, and decongestants like ephedrine, among other subgroups.[4][51][232]
Synthesis
Since the first preparation was reported in 1887,[234] numerous synthetic routes to amphetamine have been developed.[235][236] The most common route of both legal and illicit amphetamine synthesis employs a non-metal reduction known as the Leuckart reaction (method 1).[50][237] In the first step, a reaction between phenylacetone and formamide, either using additional formic acid or formamide itself as a reducing agent, yields N-formylamphetamine. This intermediate is then hydrolyzed using hydrochloric acid, and subsequently basified, extracted with organic solvent, concentrated, and distilled to yield the free base. The free base is then dissolved in an organic solvent, sulfuric acid added, and amphetamine precipitates out as the sulfate salt.[237][238]
A number of chiral resolutions have been developed to separate the two enantiomers of amphetamine.[235] For example, racemic amphetamine can be treated with d-tartaric acid to form a diastereoisomeric salt which is fractionally crystallized to yield dextroamphetamine.[239] Chiral resolution remains the most economical method for obtaining optically pure amphetamine on a large scale.[240] In addition, several enantioselective syntheses of amphetamine have been developed. In one example, optically pure (R)-1-phenyl-ethanamine is condensed with phenylacetone to yield a chiral Schiff base. In the key step, this intermediate is reduced by catalytic hydrogenation with a transfer of chirality to the carbon atom alpha to the amino group. Cleavage of the benzylic amine bond by hydrogenation yields optically pure dextroamphetamine.[240]
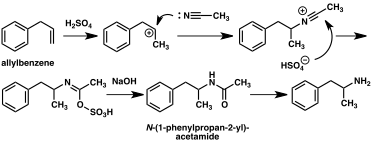
A large number of alternative synthetic routes to amphetamine have been developed based on classic organic reactions.[235][236] One example is the Friedel–Crafts alkylation of benzene by allyl chloride to yield beta chloropropylbenzene which is then reacted with ammonia to produce racemic amphetamine (method 2).[241] Another example employs the Ritter reaction (method 3). In this route, allylbenzene is reacted acetonitrile in sulfuric acid to yield an organosulfate which in turn is treated with sodium hydroxide to give amphetamine via an acetamide intermediate.[242][243] A third route starts with ethyl 3-oxobutanoate which through a double alkylation with methyl iodide followed by benzyl chloride can be converted into 2-methyl-3-phenyl-propanoic acid. This synthetic intermediate can be transformed into amphetamine using either a Hofmann or Curtius rearrangement (method 4).[244]
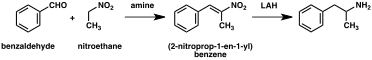
A significant number of amphetamine syntheses feature a reduction of a nitro, imine, oxime, or other nitrogen-containing functional groups.[236] In one such example, a Knoevenagel condensation of benzaldehyde with nitroethane yields phenyl-2-nitropropene. The double bond and nitro group of this intermediate is reduced using either catalytic hydrogenation or by treatment with lithium aluminium hydride (method 5).[237][245] Another method is the reaction of phenylacetone with ammonia, producing an imine intermediate that is reduced to the primary amine using hydrogen over a palladium catalyst or lithium aluminum hydride (method 6).[237]
| | |
Detection in body fluids
Amphetamine is frequently measured in urine or blood as part of a drug test for sports, employment, poisoning diagnostics, and forensics.[sources 18] Techniques such as immunoassay, which is the most common form of amphetamine test, may cross-react with a number of sympathomimetic drugs.[249] Chromatographic methods specific for amphetamine are employed to prevent false positive results.[250] Chiral separation techniques may be employed to help distinguish the source of the drug, whether prescription amphetamine, prescription amphetamine prodrugs, (e.g., selegiline), over-the-counter drug products that contain levomethamphetamine,[note 20] or illicitly obtained substituted amphetamines.[250][253][254] Several prescription drugs produce amphetamine as a metabolite, including benzphetamine, clobenzorex, famprofazone, fenproporex, lisdexamfetamine, mesocarb, methamphetamine, prenylamine, and selegiline, among others.[2][255][256] These compounds may produce positive results for amphetamine on drug tests.[255][256] Amphetamine is generally only detectable by a standard drug test for approximately 24 hours, although a high dose may be detectable for 2–4 days.[249]
For the assays, a study noted that an enzyme multiplied immunoassay technique (EMIT) assay for amphetamine and methamphetamine may produce more false positives than liquid chromatography–tandem mass spectrometry.[253] Gas chromatography–mass spectrometry (GC–MS) of amphetamine and methamphetamine with the derivatizing agent (S)-(−)-trifluoroacetylprolyl chloride allows for the detection of methamphetamine in urine.[250] GC–MS of amphetamine and methamphetamine with the chiral derivatizing agent Mosher's acid chloride allows for the detection of both dextroamphetamine and dextromethamphetamine in urine.[250] Hence, the latter method may be used on samples that test positive using other methods to help distinguish between the various sources of the drug.[250]
History, society, and culture
| Substance | Best estimate | Low estimate | High estimate |
|---|---|---|---|
| Amphetamine- type stimulants | 34.16 | 13.42 | 55.24 |
| Cannabis | 192.15 | 165.76 | 234.06 |
| Cocaine | 18.20 | 13.87 | 22.85 |
| Ecstasy | 20.57 | 8.99 | 32.34 |
| Opiates | 19.38 | 13.80 | 26.15 |
| Opioids | 34.26 | 27.01 | 44.54 |
Amphetamine was first synthesized in 1887 in Germany by Romanian chemist Lazăr Edeleanu who named it phenylisopropylamine;[234][258][259] its stimulant effects remained unknown until 1927, when it was independently resynthesized by Gordon Alles and reported to have sympathomimetic properties.[259] Amphetamine had no medical use until late 1933, when Smith, Kline and French began selling it as an inhaler under the brand name Benzedrine as a decongestant.[30] Benzedrine sulfate was introduced 3 years later and was used to treat a wide variety of medical conditions, including narcolepsy, obesity, low blood pressure, low libido, and chronic pain, among others.[52][30] During World War II, amphetamine and methamphetamine were used extensively by both the Allied and Axis forces for their stimulant and performance-enhancing effects.[234][260][261] As the addictive properties of the drug became known, governments began to place strict controls on the sale of amphetamine.[234] For example, during the early 1970s in the United States, amphetamine became a schedule II controlled substance under the Controlled Substances Act.[262][263] In spite of strict government controls, amphetamine has been used legally or illicitly by people from a variety of backgrounds, including authors,[264] musicians,[265] mathematicians,[266] and athletes.[28]
Amphetamine is still illegally synthesized today in clandestine labs and sold on the black market, primarily in European countries.[267] Among European Union (EU) member states in 2018,[update] 11.9 million adults of ages 15–64 have used amphetamine or methamphetamine at least once in their lives and 1.7 million have used either in the last year.[268] During 2012, approximately 5.9 metric tons of illicit amphetamine were seized within EU member states;[269] the "street price" of illicit amphetamine within the EU ranged from €6–38 per gram during the same period.[269] Outside Europe, the illicit market for amphetamine is much smaller than the market for methamphetamine and MDMA.[267]
Legal status
As a result of the United Nations 1971 Convention on Psychotropic Substances, amphetamine became a schedule II controlled substance, as defined in the treaty, in all 183 state parties.[31] Consequently, it is heavily regulated in most countries.[270][271] Some countries, such as South Korea and Japan, have banned substituted amphetamines even for medical use.[272][273] In other nations, such as Canada (schedule I drug),[274] the Netherlands (List I drug),[275] the United States (schedule II drug),[29] Australia (schedule 8),[276] Thailand (category 1 narcotic),[277] and United Kingdom (class B drug),[278] amphetamine is in a restrictive national drug schedule that allows for its use as a medical treatment.[267][32]
Pharmaceutical products
Several currently marketed amphetamine formulations contain both enantiomers, including those marketed under the brand names Adderall, Adderall XR, Mydayis,[note 1] Adzenys ER, Adzenys XR-ODT, Dyanavel XR, Evekeo, and Evekeo ODT. Of those, Evekeo (including Evekeo ODT) is the only product containing only racemic amphetamine (as amphetamine sulfate), and is therefore the only one whose active moiety can be accurately referred to simply as "amphetamine".[1][36][116] Dextroamphetamine, marketed under the brand names Dexedrine and Zenzedi, is the only enantiopure amphetamine product currently available. A prodrug form of dextroamphetamine, lisdexamfetamine, is also available and is marketed under the brand name Vyvanse. As it is a prodrug, lisdexamfetamine is structurally different from dextroamphetamine, and is inactive until it metabolizes into dextroamphetamine.[38][210] The free base of racemic amphetamine was previously available as Benzedrine, Psychedrine, and Sympatedrine.[2] Levoamphetamine was previously available as Cydril.[2] Many current amphetamine pharmaceuticals are salts due to the comparatively high volatility of the free base.[2][38][50] However, oral suspension and orally disintegrating tablet (ODT) dosage forms composed of the free base were introduced in 2015 and 2016, respectively.[116][279][280] Some of the current brands and their generic equivalents are listed below.
| Brand name | United States Adopted Name | (D:L) ratio | Dosage form | Marketing start date | Sources |
|---|---|---|---|---|---|
| Adderall | – | 3:1 (salts) | tablet | 1996 | [2][38] |
| Adderall XR | – | 3:1 (salts) | capsule | 2001 | [2][38] |
| Mydayis | – | 3:1 (salts) | capsule | 2017 | [281][282] |
| Adzenys ER | amphetamine | 3:1 (base) | suspension | 2017 | [283] |
| Adzenys XR-ODT | amphetamine | 3:1 (base) | ODT | 2016 | [280][284] |
| Dyanavel XR | amphetamine | 3.2:1 (base) | suspension | 2015 | [116][279] |
| Evekeo | amphetamine sulfate | 1:1 (salts) | tablet | 2012 | [36][285] |
| Evekeo ODT | amphetamine sulfate | 1:1 (salts) | ODT | 2019 | [286] |
| Dexedrine | dextroamphetamine sulfate | 1:0 (salts) | capsule | 1976 | [2][38] |
| Zenzedi | dextroamphetamine sulfate | 1:0 (salts) | tablet | 2013 | [38][287] |
| Vyvanse | lisdexamfetamine dimesylate | 1:0 (prodrug) | capsule | 2007 | [2][210][288] |
| tablet | |||||
| Xelstrym | dextroamphetamine | 1:0 (base) | patch | 2022 | [289] |
| drug | formula | molar mass [note 21] | amphetamine base [note 22] | amphetamine base in equal doses | doses with equal base content [note 23] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g/mol) | (percent) | (30 mg dose) | ||||||||
| total | base | total | dextro- | levo- | dextro- | levo- | ||||
| dextroamphetamine sulfate[291][292] | (C9H13N)2•H2SO4 | 368.49 | 270.41 | 73.38% | 73.38% | — | 22.0 mg | — | 30.0 mg | |
| amphetamine sulfate[293] | (C9H13N)2•H2SO4 | 368.49 | 270.41 | 73.38% | 36.69% | 36.69% | 11.0 mg | 11.0 mg | 30.0 mg | |
| Adderall | 62.57% | 47.49% | 15.08% | 14.2 mg | 4.5 mg | 35.2 mg | ||||
| 25% | dextroamphetamine sulfate[291][292] | (C9H13N)2•H2SO4 | 368.49 | 270.41 | 73.38% | 73.38% | — | |||
| 25% | amphetamine sulfate[293] | (C9H13N)2•H2SO4 | 368.49 | 270.41 | 73.38% | 36.69% | 36.69% | |||
| 25% | dextroamphetamine saccharate[294] | (C9H13N)2•C6H10O8 | 480.55 | 270.41 | 56.27% | 56.27% | — | |||
| 25% | amphetamine aspartate monohydrate[295] | (C9H13N)•C4H7NO4•H2O | 286.32 | 135.21 | 47.22% | 23.61% | 23.61% | |||
| lisdexamfetamine dimesylate[210] | C15H25N3O•(CH4O3S)2 | 455.49 | 135.21 | 29.68% | 29.68% | — | 8.9 mg | — | 74.2 mg | |
| amphetamine base suspension[116] | C9H13N | 135.21 | 135.21 | 100% | 76.19% | 23.81% | 22.9 mg | 7.1 mg | 22.0 mg | |
Notes
- ^ a b Adderall and other mixed amphetamine salts products such as Mydayis are not racemic amphetamine – they are a mixture composed of equal parts racemate and dextroamphetamine.
See Mixed amphetamine salts for more information about the mixture, and this section for information about the various mixtures of amphetamine enantiomers currently marketed. - ^ Synonyms and alternate spellings include: 1-phenylpropan-2-amine (IUPAC name), α-methylphenethylamine, amfetamine (International Nonproprietary Name [INN]), β-phenylisopropylamine, thyramine, and speed.[23][11][24]
- ^ Enantiomers are molecules that are mirror images of one another; they are structurally identical, but of the opposite orientation.[25]
Levoamphetamine and dextroamphetamine are also known as L-amph or levamfetamine (INN) and D-amph or dexamfetamine (INN) respectively.[23] - ^ The brand name Adderall is used throughout this article to refer to the amphetamine four-salt mixture it contains (dextroamphetamine sulfate 25%, dextroamphetamine saccharate 25%, amphetamine sulfate 25%, and amphetamine aspartate 25%). The nonproprietary name, which lists all four active constituent chemicals, is excessively lengthy.[38]
- ^ The term "amphetamines" also refers to a chemical class, but, unlike the class of substituted amphetamines,[4] the "amphetamines" class does not have a standardized definition in academic literature.[19] One of the more restrictive definitions of this class includes only the racemate and enantiomers of amphetamine and methamphetamine.[19] The most general definition of the class encompasses a broad range of pharmacologically and structurally related compounds.[19]
Due to confusion that may arise from use of the plural form, this article will only use the terms "amphetamine" and "amphetamines" to refer to racemic amphetamine, levoamphetamine, and dextroamphetamine and reserve the term "substituted amphetamines" for its structural class. - ^ The ADHD-related outcome domains with the greatest proportion of significantly improved outcomes from long-term continuous stimulant therapy include academics (≈55% of academic outcomes improved), driving (100% of driving outcomes improved), non-medical drug use (47% of addiction-related outcomes improved), obesity (≈65% of obesity-related outcomes improved), self-esteem (50% of self-esteem outcomes improved), and social function (67% of social function outcomes improved).[59]
The largest effect sizes for outcome improvements from long-term stimulant therapy occur in the domains involving academics (e.g., grade point average, achievement test scores, length of education, and education level), self-esteem (e.g., self-esteem questionnaire assessments, number of suicide attempts, and suicide rates), and social function (e.g., peer nomination scores, social skills, and quality of peer, family, and romantic relationships).[59]
Long-term combination therapy for ADHD (i.e., treatment with both a stimulant and behavioral therapy) produces even larger effect sizes for outcome improvements and improves a larger proportion of outcomes across each domain compared to long-term stimulant therapy alone.[59] - ^ Cochrane reviews are high quality meta-analytic systematic reviews of randomized controlled trials.[65]
- ^ The statements supported by the USFDA come from prescribing information, which is the copyrighted intellectual property of the manufacturer and approved by the USFDA. USFDA contraindications are not necessarily intended to limit medical practice but limit claims by pharmaceutical companies.[111]
- ^ According to one review, amphetamine can be prescribed to individuals with a history of abuse provided that appropriate medication controls are employed, such as requiring daily pick-ups of the medication from the prescribing physician.[2]
- ^ In individuals who experience sub-normal height and weight gains, a rebound to normal levels is expected to occur if stimulant therapy is briefly interrupted.[45][58][115] The average reduction in final adult height from 3 years of continuous stimulant therapy is 2 cm.[115]
- ^ Transcription factors are proteins that increase or decrease the expression of specific genes.[143]
- ^ In simpler terms, this necessary and sufficient relationship means that ΔFosB overexpression in the nucleus accumbens and addiction-related behavioral and neural adaptations always occur together and never occur alone.
- ^ NMDA receptors are voltage-dependent ligand-gated ion channels that requires simultaneous binding of glutamate and a co-agonist (D-serine or glycine) to open the ion channel.[157]
- ^ The review indicated that magnesium L-aspartate and magnesium chloride produce significant changes in addictive behavior;[134] other forms of magnesium were not mentioned.
- ^ The 95% confidence interval indicates that there is a 95% probability that the true number of deaths lies between 3,425 and 4,145.
- ^ a b The human dopamine transporter (hDAT) contains a high-affinity, extracellular, and allosteric Zn2+ (zinc ion) binding site which, upon zinc binding, inhibits dopamine reuptake, inhibits amphetamine-induced hDAT internalization, and amplifies amphetamine-induced dopamine efflux.[173][174][175][176] The human serotonin transporter and norepinephrine transporter do not contain zinc binding sites.[175]
- ^ 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[4][216] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[216][218] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[219][220]
- ^ There is substantial variation in microbiome composition and microbial concentrations by anatomical site.[221][222] Fluid from the human colon – which contains the highest concentration of microbes of any anatomical site – contains approximately one trillion (10^12) bacterial cells/ml.[221]
- ^ Enantiomers are molecules that are mirror images of one another; they are structurally identical, but of the opposite orientation.[25]
- ^ The active ingredient in some OTC inhalers in the United States is listed as levmetamfetamine, the INN and USAN of levomethamphetamine.[251][252]
- ^ For uniformity, molar masses were calculated using the Lenntech Molecular Weight Calculator[290] and were within 0.01 g/mol of published pharmaceutical values.
- ^ Amphetamine base percentage = molecular massbase / molecular masstotal. Amphetamine base percentage for Adderall = sum of component percentages / 4.
- ^ dose = (1 / amphetamine base percentage) × scaling factor = (molecular masstotal / molecular massbase) × scaling factor. The values in this column were scaled to a 30 mg dose of dextroamphetamine sulfate. Due to pharmacological differences between these medications (e.g., differences in the release, absorption, conversion, concentration, differing effects of enantiomers, half-life, etc.), the listed values should not be considered equipotent doses.
- Image legend
- ^ (Text color) Transcription factors
Reference notes
- ^ [2][26][19][27][28][29][30][31][32][33][34][35][36][37]
- ^ [2][16][27][30][36][39][40]
- ^ [16][27][28][29][33][41][42][43][44][45][46][47][48]
- ^ [49][50][51]
- ^ a b [71][37][75][72][77]
- ^ [1][29][41][115][116][117]
- ^ [118][119][120][121]
- ^ [29][112][118][120]
- ^ [33][29][41][122]
- ^ a b [137][138][139][140][159]
- ^ [135][137][136][144][145]
- ^ [136][147][148][149]
- ^ [24][29][41][163][165]
- ^ [53][167][170][171]
- ^ a b [178][182][186][187][188][189][190]
- ^ [39][178][186][187][191][196]
- ^ a b [3][4][5][6][7][12][216][217]
- ^ [28][246][247][248]
References
- ^ a b c d e f g h i j k Stahl SM (March 2017). "Amphetamine (D,L)". Prescriber's Guide: Stahl's Essential Psychopharmacology (6th ed.). Cambridge, United Kingdom: Cambridge University Press. pp. 45–51. ISBN 9781108228749. Retrieved 5 August 2017.
- ^ a b c d e f g h i j k l m Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". Journal of Psychopharmacology. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
The intravenous use of d-amphetamine and other stimulants still pose major safety risks to the individuals indulging in this practice. Some of this intravenous abuse is derived from the diversion of ampoules of d-amphetamine, which are still occasionally prescribed in the UK for the control of severe narcolepsy and other disorders of excessive sedation. ... For these reasons, observations of dependence and abuse of prescription d-amphetamine are rare in clinical practice, and this stimulant can even be prescribed to people with a history of drug abuse provided certain controls, such as daily pick-ups of prescriptions, are put in place (Jasinski and Krishnan, 2009b).
- ^ a b c d e f g h i j k l m n o p q r s "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ^ a b c d e f g h i Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ a b Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. doi:10.1016/S0021-9258(19)43051-2. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ a b c Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ a b Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ Anvisa (31 March 2023). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 4 April 2023). Archived from the original on 3 August 2023. Retrieved 3 August 2023.
- ^ a b Patel VB, Preedy VR, eds. (2022). Handbook of Substance Misuse and Addictions. Cham: Springer International Publishing. p. 2006. doi:10.1007/978-3-030-92392-1. ISBN 978-3-030-92391-4.
Amphetamine is usually consumed via inhalation or orally, either in the form of a racemic mixture (levoamphetamine and dextroamphetamine) or dextroamphetamine alone (Childress et al. 2019). In general, all amphetamines have high bioavailability when consumed orally, and in the specific case of amphetamine, 90% of the consumed dose is absorbed in the gastrointestinal tract, with no significant differences in the rate and extent of absorption between the two enantiomers (Carvalho et al. 2012; Childress et al. 2019). The onset of action occurs approximately 30 to 45 minutes after consumption, depending on the ingested dose and on the degree of purity or on the concomitant consumption of certain foods (European Monitoring Centre for Drugs and Drug Addiction 2021a; Steingard et al. 2019). It is described that those substances that promote acidification of the gastrointestinal tract cause a decrease in amphetamine absorption, while gastrointestinal alkalinization may be related to an increase in the compound's absorption (Markowitz and Patrick 2017).
- ^ a b c d e f g h i Wishart DS, Djombou Feunang Y, Guo AC, Lo EJ, Marcu A, Grant JR, et al. "Amphetamine | DrugBank Online". DrugBank. 5.0.
- ^ a b c d Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ^ "Pharmacology". amphetamine/dextroamphetamine. Medscape - WebMD. Retrieved 21 January 2016.
Onset of action: 30–60 min
- ^ a b c Millichap JG (2010). "Chapter 9: Medications for ADHD". In Millichap JG (ed.). Attention Deficit Hyperactivity Disorder Handbook: A Physician's Guide to ADHD (2nd ed.). New York: Springer. p. 112. ISBN 9781441913968.
Table 9.2 Dextroamphetamine formulations of stimulant medication
Dexedrine [Peak:2–3 h] [Duration:5–6 h] ...
Adderall [Peak:2–3 h] [Duration:5–7 h]
Dexedrine spansules [Peak:7–8 h] [Duration:12 h] ...
Adderall XR [Peak:7–8 h] [Duration:12 h]
Vyvanse [Peak:3–4 h] [Duration:12 h] - ^ Brams M, Mao AR, Doyle RL (September 2008). "Onset of efficacy of long-acting psychostimulants in pediatric attention-deficit/hyperactivity disorder". Postgraduate Medicine. 120 (3): 69–88. doi:10.3810/pgm.2008.09.1909. PMID 18824827. S2CID 31791162.
- ^ a b c d "Adderall- dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate tablet". DailyMed. Teva Pharmaceuticals USA, Inc. 8 November 2019. Retrieved 22 December 2019.
- ^ a b c d e f "Metabolism/Pharmacokinetics". Amphetamine. Hazardous Substances Data Bank. United States National Library of Medicine – Toxicology Data Network. Archived from the original on 2 October 2017. Retrieved 2 October 2017.
Duration of effect varies depending on agent and urine pH. Excretion is enhanced in more acidic urine. Half-life is 7 to 34 hours and is, in part, dependent on urine pH (half-life is longer with alkaline urine). ... Amphetamines are distributed into most body tissues with high concentrations occurring in the brain and CSF. Amphetamine appears in the urine within about 3 hours following oral administration. ... Three days after a dose of (+ or -)-amphetamine, human subjects had excreted 91% of the (14)C in the urine
- ^ a b c d e f g h i j Mignot EJ (October 2012). "A practical guide to the therapy of narcolepsy and hypersomnia syndromes". Neurotherapeutics. 9 (4): 739–752. doi:10.1007/s13311-012-0150-9. PMC 3480574. PMID 23065655.
At the pathophysiological level, it is now clear that most narcolepsy cases with cataplexy, and a minority of cases (5–30 %) without cataplexy or with atypical cataplexy-like symptoms, are caused by a lack of hypocretin (orexin) of likely an autoimmune origin. In these cases, once the disease is established, the majority of the 70,000 hypocretin-producing cells have been destroyed, and the disorder is irreversible. ...
Amphetamines are exceptionally wake-promoting, and at high doses also reduce cataplexy in narcoleptic patients, an effect best explained by its action on adrenergic and serotoninergic synapses. ...
The D-isomer is more specific for DA transmission and is a better stimulant compound. Some effects on cataplexy (especially for the L-isomer), secondary to adrenergic effects, occur at higher doses. ...
Numerous studies have shown that increased dopamine release is the main property explaining wake-promotion, although norepinephrine effects also contribute. - ^ a b c d e Yoshida T (1997). "Chapter 1: Use and Misuse of Amphetamines: An International Overview". In Klee H (ed.). Amphetamine Misuse: International Perspectives on Current Trends. Amsterdam, Netherlands: Harwood Academic Publishers. p. 2. ISBN 9789057020810.
Amphetamine, in the singular form, properly applies to the racemate of 2-amino-1-phenylpropane. ... In its broadest context, however, the term [amphetamines] can even embrace a large number of structurally and pharmacologically related substances.
- ^ "Density". Amphetamine. United States National Library of Medicine – National Center for Biotechnology Information. PubChem Compound Database. 5 November 2016. Retrieved 9 November 2016.
- ^ Amphetamine. American Chemical Society. CAS Common Chemistry. Retrieved 25 October 2022.
- ^ a b "Chemical and Physical Properties". Amphetamine. United States National Library of Medicine – National Center for Biotechnology Information. PubChem Compound Database. Retrieved 13 October 2013.
- ^ a b c "Compound Summary". Amphetamine. United States National Library of Medicine – National Center for Biotechnology Information. PubChem Compound Database. 11 April 2015. Retrieved 17 April 2015.
- ^ a b Greene SL, Kerr F, Braitberg G (October 2008). "Review article: amphetamines and related drugs of abuse". Emergency Medicine Australasia. 20 (5): 391–402. doi:10.1111/j.1742-6723.2008.01114.x. PMID 18973636. S2CID 20755466.
- ^ a b "Enantiomer". IUPAC Compendium of Chemical Terminology. International Union of Pure and Applied Chemistry. IUPAC Goldbook. 2009. doi:10.1351/goldbook.E02069. ISBN 9780967855097. Archived from the original on 17 March 2013. Retrieved 14 March 2014.
One of a pair of molecular entities which are mirror images of each other and non-superposable.
- ^ Rasmussen N (January 2015). Taba P, Lees A, Sikk K (eds.). "Amphetamine-Type Stimulants: The Early History of Their Medical and Non-Medical Uses". International Review of Neurobiology. The Neuropsychiatric Complications of Stimulant Abuse. 120. Academic Press: 9–25. doi:10.1016/bs.irn.2015.02.001. PMID 26070751.
- ^ a b c d e f g h i j Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York, US: McGraw-Hill Medical. pp. 318, 321. ISBN 9780071481274.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in normal subjects and those with ADHD. ... stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks ... through indirect stimulation of dopamine and norepinephrine receptors. ...
Beyond these general permissive effects, dopamine (acting via D1 receptors) and norepinephrine (acting at several receptors) can, at optimal levels, enhance working memory and aspects of attention. - ^ a b c d e f g Liddle DG, Connor DJ (June 2013). "Nutritional supplements and ergogenic AIDS". Primary Care: Clinics in Office Practice. 40 (2): 487–505. doi:10.1016/j.pop.2013.02.009. PMID 23668655.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training ...
Physiologic and performance effects
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an "Adderall XR- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate capsule, extended release". DailyMed. Shire US Inc. 17 July 2019. Retrieved 22 December 2019.
- ^ a b c d Rasmussen N (July 2006). "Making the first anti-depressant: amphetamine in American medicine, 1929–1950". Journal of the History of Medicine and Allied Sciences. 61 (3): 288–323. doi:10.1093/jhmas/jrj039. PMID 16492800. S2CID 24974454.
However the firm happened to discover the drug, SKF first packaged it as an inhaler so as to exploit the base's volatility and, after sponsoring some trials by East Coast otolaryngological specialists, began to advertise the Benzedrine Inhaler as a decongestant in late 1933.
- ^ a b "Convention on psychotropic substances". United Nations Treaty Collection. United Nations. Archived from the original on 31 March 2016. Retrieved 11 November 2013.
- ^ a b Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, et al. (January 2008). "Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature". Journal of the American Academy of Child & Adolescent Psychiatry. 47 (1): 21–31. doi:10.1097/chi.0b013e31815a56f1. PMID 18174822.
Stimulant misuse appears to occur both for performance enhancement and their euphorogenic effects, the latter being related to the intrinsic properties of the stimulants (e.g., IR versus ER profile) ...
Although useful in the treatment of ADHD, stimulants are controlled II substances with a history of preclinical and human studies showing potential abuse liability. - ^ a b c Montgomery KA (June 2008). "Sexual desire disorders". Psychiatry. 5 (6): 50–55. PMC 2695750. PMID 19727285.
- ^ "Amphetamine". Medical Subject Headings. United States National Library of Medicine. Retrieved 16 December 2013.
- ^ "Guidelines on the Use of International Nonproprietary Names (INNS) for Pharmaceutical Substances". World Health Organization. 1997. Archived from the original on 9 January 2015. Retrieved 1 December 2014.
In principle, INNs are selected only for the active part of the molecule which is usually the base, acid or alcohol. In some cases, however, the active molecules need to be expanded for various reasons, such as formulation purposes, bioavailability or absorption rate. In 1975 the experts designated for the selection of INN decided to adopt a new policy for naming such molecules. In future, names for different salts or esters of the same active substance should differ only with regard to the inactive moiety of the molecule. ... The latter are called modified INNs (INNMs).
- ^ a b c d e f g h i "Evekeo- amphetamine sulfate tablet". DailyMed. Arbor Pharmaceuticals, LLC. 14 August 2019. Retrieved 22 December 2019.
- ^ a b c d e f Rodan SC, Bryant E, Le A, Maloney D, Touyz S, McGregor IS, et al. (July 2023). "Pharmacotherapy, alternative and adjunctive therapies for eating disorders: findings from a rapid review". Journal of Eating Disorders. 11 (1): 112. doi:10.1186/s40337-023-00833-9. PMC 10327007. PMID 37415200.
LDX is commonly used in the treatment of ADHD, and is the only treatment for BED that is currently approved by the Food and Drug Administration (FDA) and the Therapeutic Goods Administration (TGA). LDX, like all amphetamine stimulants, has direct appetite suppressant effects that may be therapeutically useful in BED, although long-term neuroadaptations in dopaminergic and noradrenergic systems caused by LDX may also be relevant, leading to improved regulation of eating behaviours, attentional processes and goal-directed behaviours. ...
Evidently, there is a substantial volume of trials with high-quality evidence supporting the efficacy of LDX in reducing binge eating frequency in treatment of adults with moderate to severe BED at 50–70 mg/day. - ^ a b c d e f g h "National Drug Code Amphetamine Search Results". National Drug Code Directory. United States Food and Drug Administration. Archived from the original on 16 December 2013. Retrieved 16 December 2013.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ^ a b c d e Grandy DK, Miller GM, Li JX (February 2016). ""TAARgeting Addiction"-The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference". Drug and Alcohol Dependence. 159: 9–16. doi:10.1016/j.drugalcdep.2015.11.014. PMC 4724540. PMID 26644139.
When considered together with the rapidly growing literature in the field a compelling case emerges in support of developing TAAR1-selective agonists as medications for preventing relapse to psychostimulant abuse.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC (eds.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York, US: McGraw-Hill. ISBN 9780071624428.
- ^ a b c d e Shoptaw SJ, Kao U, Ling W (January 2009). Shoptaw SJ, Ali R (eds.). "Treatment for amphetamine psychosis". Cochrane Database of Systematic Reviews. 2009 (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMC 7004251. PMID 19160215.
A minority of individuals who use amphetamines develop full-blown psychosis requiring care at emergency departments or psychiatric hospitals. In such cases, symptoms of amphetamine psychosis commonly include paranoid and persecutory delusions as well as auditory and visual hallucinations in the presence of extreme agitation. More common (about 18%) is for frequent amphetamine users to report psychotic symptoms that are sub-clinical and that do not require high-intensity intervention ...
About 5–15% of the users who develop an amphetamine psychosis fail to recover completely (Hofmann 1983) ...
Findings from one trial indicate use of antipsychotic medications effectively resolves symptoms of acute amphetamine psychosis.
psychotic symptoms of individuals with amphetamine psychosis may be due exclusively to heavy use of the drug or heavy use of the drug may exacerbate an underlying vulnerability to schizophrenia. - ^ a b c d e Bramness JG, Gundersen ØH, Guterstam J, Rognli EB, Konstenius M, Løberg EM, et al. (December 2012). "Amphetamine-induced psychosis—a separate diagnostic entity or primary psychosis triggered in the vulnerable?". BMC Psychiatry. 12: 221. doi:10.1186/1471-244X-12-221. PMC 3554477. PMID 23216941.
In these studies, amphetamine was given in consecutively higher doses until psychosis was precipitated, often after 100–300 mg of amphetamine ... Secondly, psychosis has been viewed as an adverse event, although rare, in children with ADHD who have been treated with amphetamine
- ^ a b c Greydanus D. "Stimulant Misuse: Strategies to Manage a Growing Problem" (PDF). American College Health Association (Review Article). ACHA Professional Development Program. p. 20. Archived from the original (PDF) on 3 November 2013. Retrieved 2 November 2013.
- ^ a b c d e f g Huang YS, Tsai MH (July 2011). "Long-term outcomes with medications for attention-deficit hyperactivity disorder: current status of knowledge". CNS Drugs. 25 (7): 539–554. doi:10.2165/11589380-000000000-00000. PMID 21699268. S2CID 3449435.
Several other studies,[97-101] including a meta-analytic review[98] and a retrospective study,[97] suggested that stimulant therapy in childhood is associated with a reduced risk of subsequent substance use, cigarette smoking and alcohol use disorders. ... Recent studies have demonstrated that stimulants, along with the non-stimulants atomoxetine and extended-release guanfacine, are continuously effective for more than 2-year treatment periods with few and tolerable adverse effects. The effectiveness of long-term therapy includes not only the core symptoms of ADHD, but also improved quality of life and academic achievements. The most concerning short-term adverse effects of stimulants, such as elevated blood pressure and heart rate, waned in long-term follow-up studies. ... The current data do not support the potential impact of stimulants on the worsening or development of tics or substance abuse into adulthood. In the longest follow-up study (of more than 10 years), lifetime stimulant treatment for ADHD was effective and protective against the development of adverse psychiatric disorders.
- ^ a b Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015)